|
|
 |
Back to 2011 Program
Precise Control of Osteogenesis for Craniofacial Defect Repair: The Role of Direct Osteoprogenitor Contact in BMP-2-Based Bioprinting
Darren M. Smith, MD1, James J. Cray, PhD1, Lee E. Weiss, PhD2, Elmer K. Dai Fei, BS2, Sameer Shakir, BS1, S. Alex Rottgers, MD1, Joseph E. Losee, MD1, Philip G. Campbell, PhD2, Gregory M. Cooper, PhD1.
1University of Pittsburgh, Pittsburgh, PA, USA, 2Carnegie Mellon University, Pittsburgh, PA, USA.
BACKGROUND:
Success with bone morphogenetic protein-2 (BMP-2) has been widely reported in the osseous reconstruction of large calvarial defects. These efforts have required enormous doses of BMP-2 and are not sufficiently refined to facilitate the detail-oriented repair required for intricate craniofacial structures. We have previously shown that inkjet-based bioprinting technologies allow for precisely customized low-dose protein patterns to be immobilized on biocompatible scaffolds with resultant spatially regulated osteogenesis. Here, we investigate the importance of direct contact between bioprinted BMP-2 and a plentiful source of osteoprogenitors, the dura mater, in mediating healing of calvarial defects.
METHODS:
Standardized 5mm osseous defects were trephinated in mouse parietal bones (N=8). Meticulous care was taken under a surgical microscope to preserve the underlying dura mater. Circular acellular dermal matrix (ADM) implants were prepared such that one semicircle of one face per implant was printed with BMP-2 bio-ink (see Figure below). These implants were then placed ink-towards (N=3) or ink-away (N=5) from the underlying dura mater. After four weeks, osteogenesis was assessed in each of the four possible positions (BMP-2-printed area towards dura, BMP-2-printed area away from dura, unprinted area towards dura, and unprinted area away from dura) by faxitron and quantified by threshold in Adobe Photoshop (San Jose, CA) and TGS Amira (San Diego, CA).
RESULTS:
The BMP-2-printed portion of the ADM generated bone covering an average of 66.5% of its surface area when it was face-down (printed surface directly abutting dura mater). By comparison, the BMP-2-printed portion of the ADM generated bone covering an average of only 21.3% of its surface area when it was face-up (printed surface away from dura). Similarly, the unprinted portion of the ADM generated an average of only 18.6% osseous coverage when face-down and 18.4% when face-up.
CONCLUSIONS:
We have previously shown that inkjet-based bioprinting has the potential to significantly enhance the role of regenerative therapies in craniofacial surgery. This technology affords the precise control of osteogenesis necessary to reconstruct this region’s intricate anatomical architecture. In the present study, we demonstrate that direct apposition of BMP-2-printed ADM to a source of osteoprogenitor cells (in this case dura mater) is necessary for bio-ink-directed osteogenesis to occur. These results have important implications for the design of more complex bioprinted osseous structures. 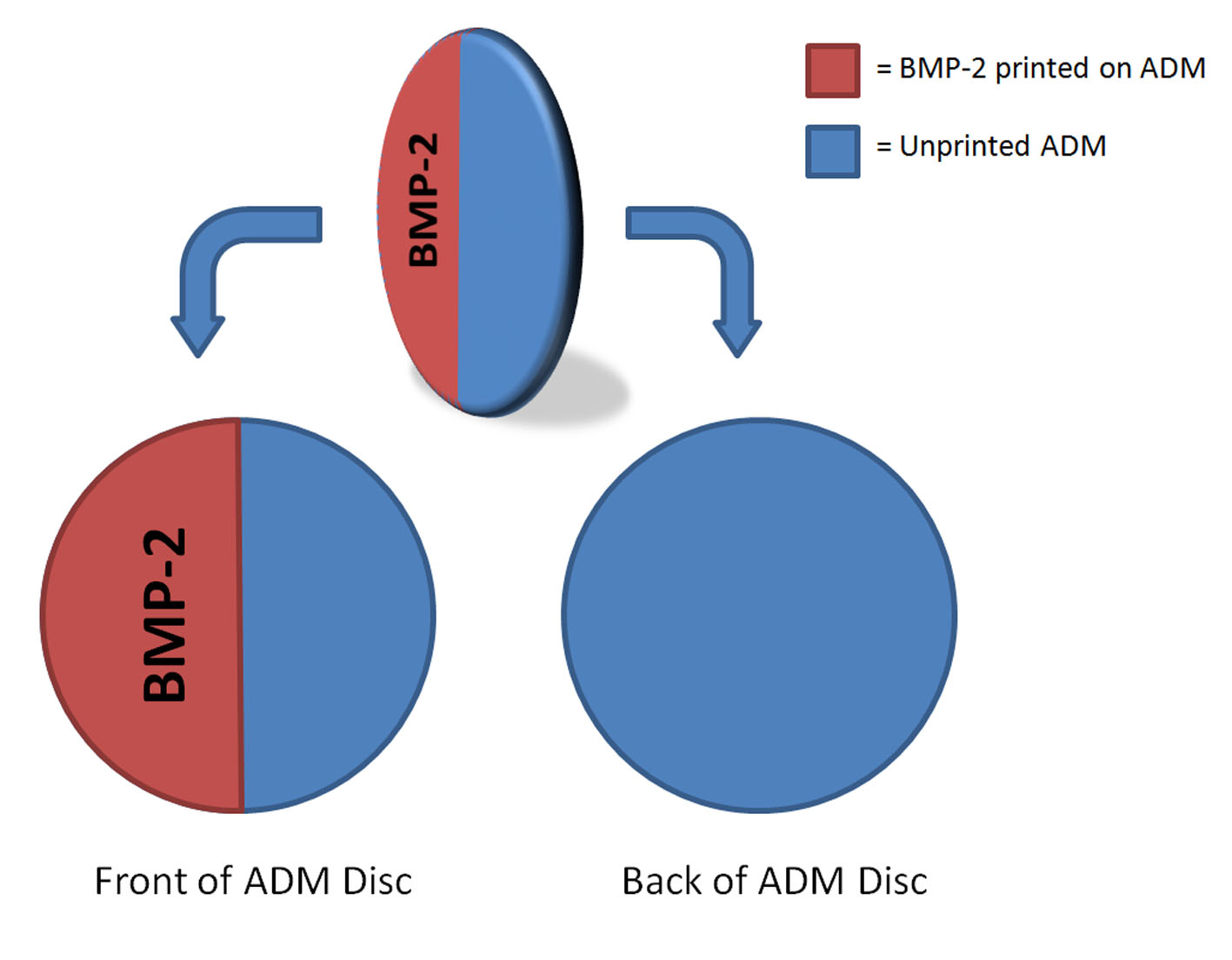
Back to 2011 Program
|








