|
|
 |
Back to 2011 Program
Chondrogenesis by Bone Marrow-Derived Mesenchymal Stem Cells Grown in Chondrocyte Conditioned Media for Possible Ear Reconstruction
Xing Zhao, M.D. & M.S.1, Nathaniel Hwang, Ph.D.2, David Bichara, M.D.1, Irina Pomerantseva, M.D. & Ph.D.1, Cathryn Sunback, ScD.1, Chris Bowley3, Scott Goldman3, Joseph Vacanti, M.D.1, Daniel Anderson, Ph.D.2, Mark Randolph, M.A.S.1, Michael Yaremchuk, M.D.1.
1Massachusetts General Hospital, Boston, MA, USA, 2Massachusetts Institute of Technology, Cambridge, MA, USA, 3Kensey Nash Corporation, Exton, PA, USA.
Background: In patients requiring auricular reconstruction, strategies for engineering ear cartilage are severely limited by the availability of auricular chondrocytes, and other anatomical sources of chondrocytes such as costal or nasal cartilage may not yield sufficient numbers of cells for an entire ear replacement. A plentiful supply of chondroprogenitor cells obtained by minimally invasive means could overcome this limitation. The aims of this study were: 1) to determine if auricular cartilage can be engineered using sheep bone marrow-derived mesenchymal stem cells (BMSCs) and a fibrous collagen scaffold; and 2) to determine if conditioned medium collected from cultured auricular chondrocytes can promote chondrogenic differentiation of BMSCs.
Methods: Auricular chondrocytes from sheep were isolated and grown in culture in standard chondrocyte medium with fetal bovine serum, ascorbic acid and non-essential amino acids. Bone marrow was aspirated from the iliac crest; BMSCs were isolated based on their ability to adhere to tissue culture plastic. Chondrocyte conditioned medium (CCM) was collected from the chondrocyte cultures. BMSCs were expanded in either CCM or standard medium (SM) to passage 2 at which time the cells were seeded onto collagen scaffolds provided by Kensey Nash Corp. Five million cells were seeded onto each collagen scaffold (5 mm diameter x 2 mm). The seeded constructs were cultured for two weeks with or without transforming growth factor-β3 (TGF-β3). After the culture period, the constructs were implanted subcutaneously in nude mice for 6 and 12 weeks. The specimens were evaluated with RT-PCR, histologically, immunohistochemically, and biochemically. Four groups were analyzed: G1- CCM+TGFβ3, G2- SM+TGFβ3, G3- CCM without TGFβ3, and G4- SM without TGFβ3.
Results: RT-PCR results showed upregulation of collagen type II (Fig. 1) in the constructs during the 14 days in vitro culture in G1 grown in CCM+ TGFβ3 and in G3 in CCM alone (Fig. 1). After 12 weeks in vivo, G1 and G3 implants showed neocartilage formation with positive type II collagen, elastin, and glycosaminoglycan production as evidenced with safranin-O and toluidine blue stains (Fig. 2). No cartilage matrix was observed in specimens grown in standard media, with TGFβ3 in G2 or without in G4. Biochemical analysis showed the consistent finding with histological results (Fig. 3).
Conclusion: Chondrocyte conditioned medium (CCM) was effective in inducing chondrogenesis by BMSCs. CCM had a stronger influence in promoting chondrogenesis than the addition of TGFβ3. Efficient chondrogenic differentiation of MSCs could provide sufficient cell population for adult size human ear-shaped scaffold. 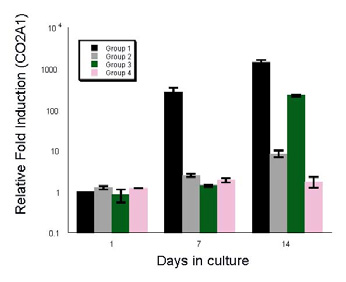 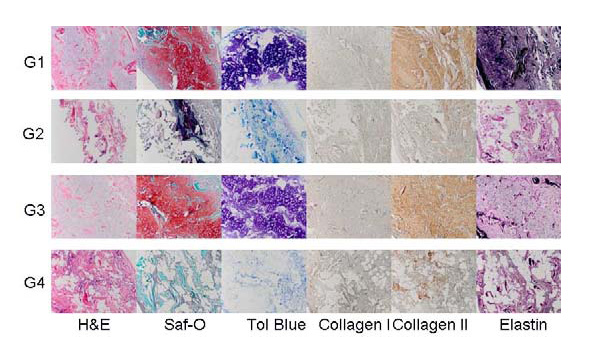 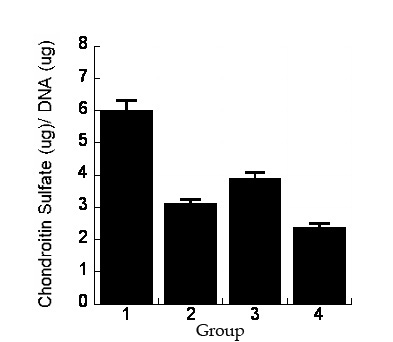
Back to 2011 Program
|










