|
|
 |
Back to 2011 Posters
Rational Design of Microfabricated Templates for Optimization of Neovascularization of Acellular Templates in vivo
Alyssa J. Reiffel, MD1, Ying Zheng, PhD2, Peter W. Henderson, MD, MBA1, Nak W. Choi, PhD2, Lawrence J. Bonassar, PhD3, Abraham D. Stroock, PhD2, Jason A. Spector, MD, FACS1.
1Weill Cornell Medical College, New York, NY, USA, 2Cornell University, School of Chemical and Biomolecular Engineering, Ithaca, NY, USA, 3Cornell University, Department of Biomedical Engineering, Sibley School of Mechanical and Aerospace Engineering, Ithaca, NY, USA.
BACKGROUND: Tissue-engineered dermal replacements require host vascular invasion to achieve permanent integration, a process that can span several weeks and as a result places the patient at risk for pain, infection, and necessitates multiple dressing changes. We hypothesized that the “rational” design of a biocompatible, biodegradable scaffold with specific pore size and containing a microchannel network would drive long-range guidance of cells through the full-thickness of the matrix. We further hypothesized that such constructs would demonstrate more rapid cellular and vascular invasion than commercially available tissue regeneration scaffolds.
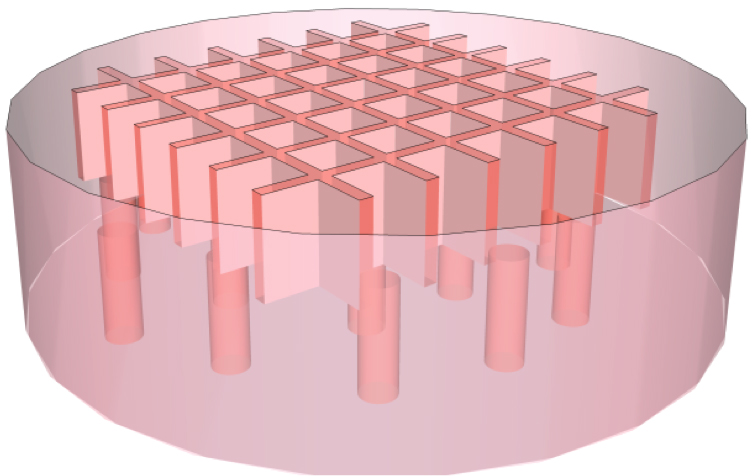
METHODS: Acellular 2% collagen type I hydrogel 8mm scaffold disks were fabricated with microphotolithographic techniques to be poreless or to contain 100, 200 or 400µm pores interconnected with a microfluidic lateral network (Figure 1). These microstructured tissue templates (MTTs) were subcutaneously implanted on the dorsa of C57BL/6 mice and harvested after 3, 7, or 14 days. Specimens were analyzed with hematoxylin & eosin (H&E), immunohistochemical (IHC) staining for CD31 (PECAM-1), α-smooth muscle actin (α-SMA), and vascular endothelial growth factor (VEGF), and counterstained with 4',6-diamidino-2-phenylindole (DAPI). Spatial and temporal cell and blood vessel density distributions were quantified using DAPI and CD31 cell counts, respectively. For comparison, Integra® grafts were similarly implanted in the dorsa of mice, and explanted and analyzed as above.
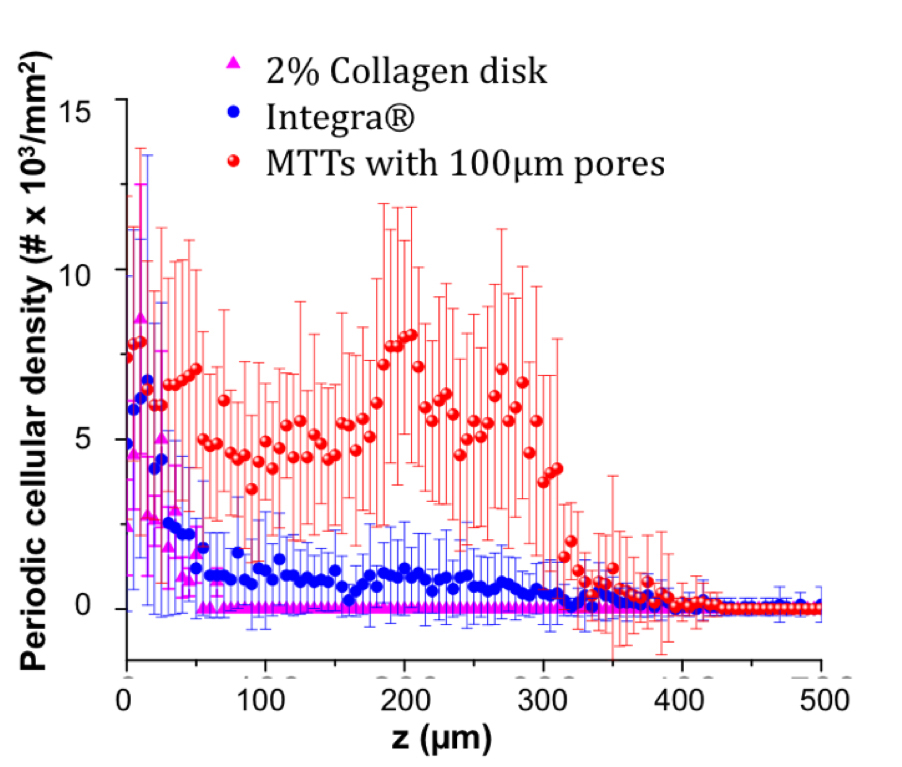
RESULTS: On histological examination, cells had entered the full pore-depth and into the lateral network of all pore-containing scaffolds by 3d. No cells were seen within the bulk of poreless scaffolds by 14d. Lateral invasion began from 100µm pores by 7d. By this time, 400µm pores had largely collapsed and few cells had traversed pore boundaries. By 14d, CD31 and α-SMA IHC revealed the presence of blood vessels within and adjacent to the 100µm pores and confluent lateral network. α-SMA staining co-localized to these vessels, suggesting perivascular cell recruitment. Areas of VEGF positivity corresponded to areas of neovascularization. Cell density distribution within 100, 200, and 400μm was high within pores by 3d, slightly decreased by 7d, and greatest within and lateral to pores by 14d. Similarly, blood vessel density rose within and lateral to pores by 14d. Overall, scaffolds containing 100µm pores recruited significantly more cells by 14d than scaffolds with pores of larger diameter. Lastly, microfeatured scaffolds recruited significantly more cells than either Integra® or featureless collagen disks after 14d (Figure 2).
CONCLUSIONS: This study not only proves that our rationally designed microfabricated scaffold is more rapidly and thoroughly vascularized than Integra® (a widely applied, commercially available dermal replacement scaffold), but it further provides crucial information regarding the geometric and chemical cues that drive vertical and lateral cellular invasion into hydrogel scaffolds.
Back to 2011 Posters
|









