|
|
 |
Back to Annual Meeting Program
Paracrine Instruction of Vasculogenesis within Naturally Derived, Biodegradable Hybrid Hydrogel Scaffolds
Alyssa J. Reiffel, MD, Justin L. Perez, BS, Karina A. Hernandez, DO, Natalia Fullerton, MD, Jason A. Spector, MD, FACS.
Weill Cornell Medical College, New York, NY, USA.
BACKGROUND: Despite their frequent use, commercially available hydrogel-based tissue regeneration templates are limited not only by their low tensile strength (and resultant poor surgical handling characteristics), but also by the rate at which they become vascularized. Any means of accelerating this process could potentially result in meaningful clinical advances in patient care. In previous work, we have demonstrated that naturally-derived, biodegradable hybrid hydrogel scaffolds fabricated from a 10:1 w/w combination of alginate and type I collagen allow for maximal human umbilical vein endothelial cell (HUVEC) adherence and invasion compared with other substrate combinations in an in vitro wound healing model. We next investigated the potential for induction of endothelial tubule formation within the “optimal” hydrogel by paracrine instruction via co-culture techniques.
METHODS: Hybrid hydrogel scaffolds were fabricated and Arg-Gly-Asp (RGD)-modified as before, seeded with 3.0x10^4, 6.0x10^4, 1.5x10^5, or 3.0x10^5 neonatal human dermal fibroblasts (HDFn) or green fluorescent protein-labeled human aortic smooth muscle cells (HASMC) and cultured in the appropriate media. On the third day of culture, scaffolds were seeded with 3.0x10^5 HUVECS and media was exchanged for HUVEC media. Scaffolds were labeled with DiI-Ac-LDL and DAPI, and fixed on culture day 17. The three-dimensional organization of the resulting cellular structures was evaluated using confocal fluorescent microscopy.
RESULTS: After 15 days of HDFn co-culture, few HUVECS remained in situ on hydrogel scaffolds. In contrast, the majority of cells present were DAPI-positive, DiI-negative fibroblasts. In contrast, co-culture with HAMSCs resulted distinctive changes in HUVEC phenotype with increasing HAMSC proportions (Figure 1). When cultured at a 10:1 ratio with HASMCs, HUVECs remained as single isolated cells and did not coalesce to form tubules. At a 5:1 ratio, HUVECS demonstrated mixed phenotypes including isolated single cells as well as the occasional formation of small tubule-like structures. Further increasing the HASMC ratio to 2:1 and 1:1 resulted in a significant increase in aggregation to larger and more organized networks of lumen-containing tubules. Of note, GFP-positive, DiI-Ac-LDL-negative HASMCS comprised a significant structural component of these networks (Figure 2) and were in direct physical contact with HUVECS
CONCLUSIONS: Although others have reported that endothelial cell co-culture with dermal fibroblasts stimulates tubule formation within hydrogels, in our study the desired results were only seen when co-culture with smooth muscle cells of vascular origin was performed. However, it is not surprising that vascular smooth muscle cells, cells closely related to and possibly even derived from pericytes, and not fibroblasts of dermal origin, would be more likely to guide the complex process of vasculogenesis. These findings serve to guide our future endeavors towards fabrication of pre-vascularized tissue constructs. 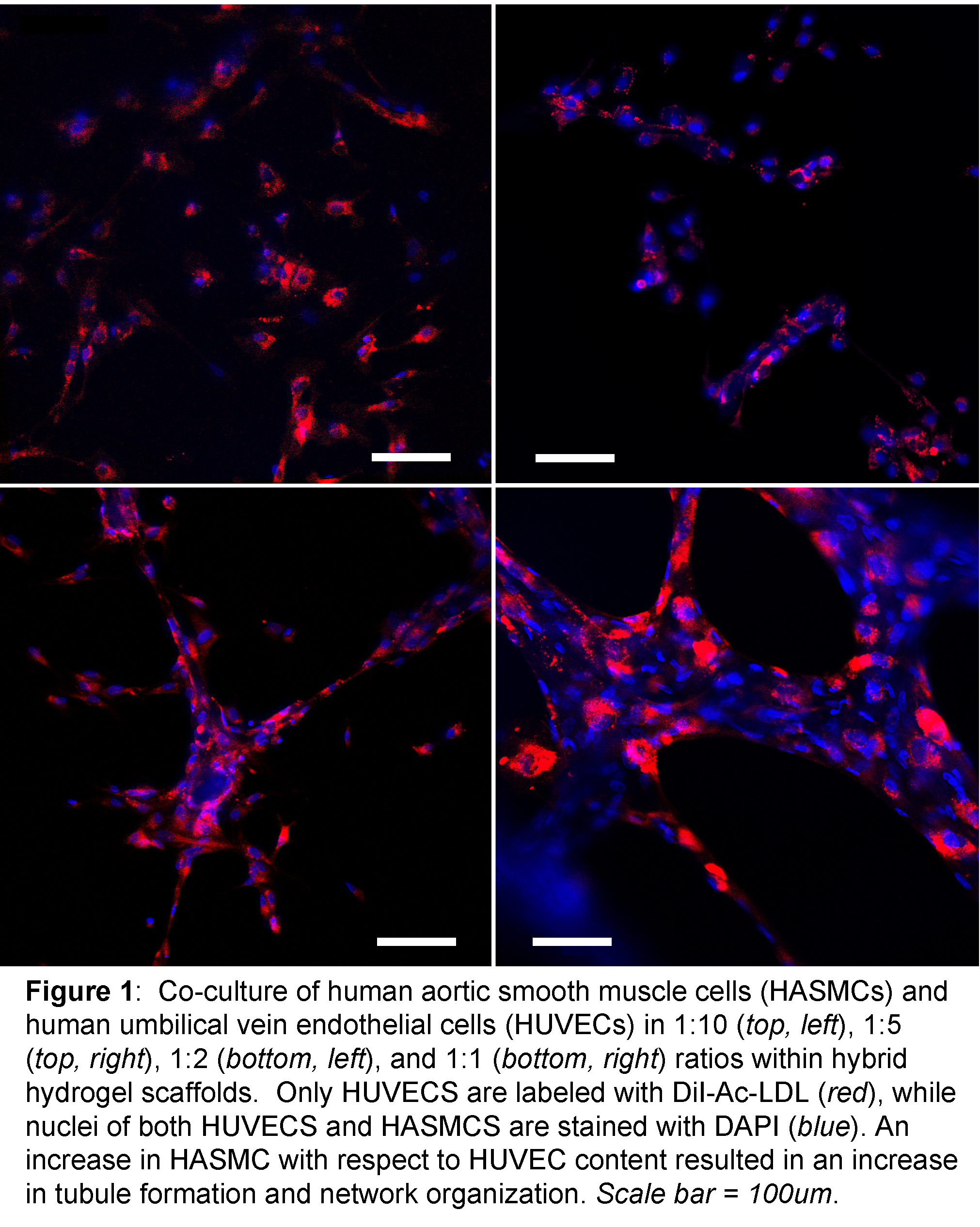 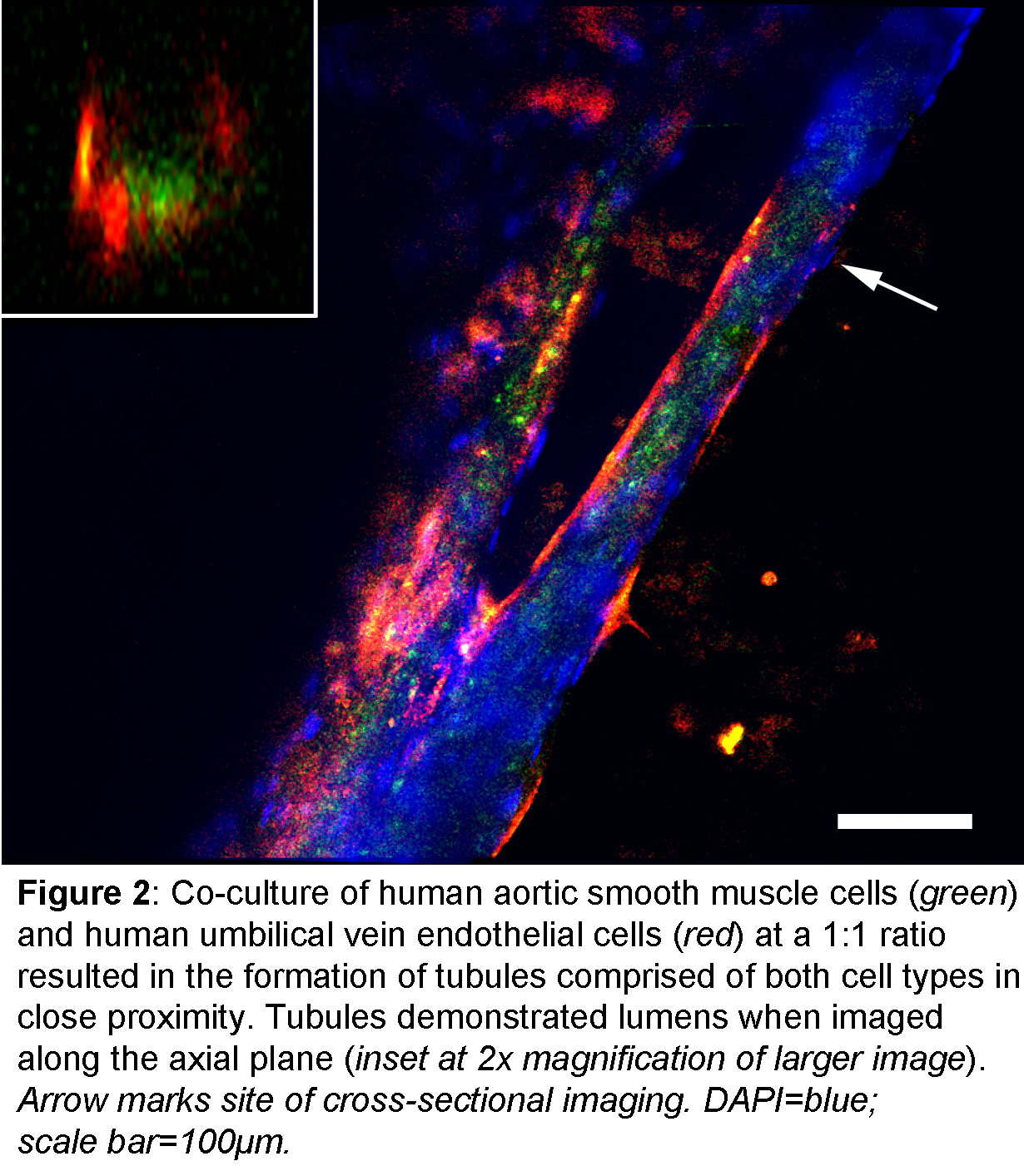
Back to Annual Meeting Program
|








