|
|
 |
Back to Annual Meeting Program
Co-Stimulatory Blockade And Donor Bone Marrow Cell Infusion For Targeted Immunomodulation In A Complete MHC-Mismatched Swine Hind Limb Transplant Model - A Large Animal Translational Study
Zuhaib Ibrahim, MD1, Galen Wachtman2, Eric Wimmers, MD1, Gabriel Brat, MD1, Johanna Grahammer, BS1, Kate Buretta, BS1, Joani Christensen, BS1, Nance Yuan, BA1, Georg Furtmuller, MD1, Vijay Gorantla2, Stefan Schneeberger, MD1, Jaimie T. Shores, MD1, Damon Cooney, MD, PhD1, Justin M. Sacks, MD1, WP Andrew Lee, MD1, Gerald Brandacher, MD1.
1Johns Hopkins University School of Medicine, Baltimore, MD, USA, 2University of Pittsburgh Medical Center, Pittsburgh, PA, USA.
BACKGROUND:
Reconstructive transplantation represents a bona fide option for select patients with devastating tissue loss, which could better restore the appearance, anatomy, and function than any other conventional treatment currently available. Despite favorable outcomes, broad clinical application of reconstructive transplantation is limited by the potential side effects of chronic multidrug immunosuppression. We have developed a method of targeted immunomodulation utilizing donor bone marrow (BM) infusion combined with co-stimulation blockade in a preclinical model of complete MHC-mismatched swine heterotopic hind limb transplantation.
METHODS:
MGH miniature swine (n=24) underwent heterotopic hind limb transplantation containing intact vascularized BM and received a short course of 30 days of Tacrolimus monotherapy. Animals in BM infusion group received 60 million cells/Kg of whole unmodified BM. Group 1 (control) received no treatment. Group 2 received 30 days of Tacrolimus only; Group 3 received irradiation and Tacrolimus; Group 4 received irradiation, BM infusion and Tacrolimus; Group 5 received Tacrolimus and CTLA4-lg; and Group 6 received BM infusion, Tacrolimus and CTLA4Ig fusion protein.
RESULTS:
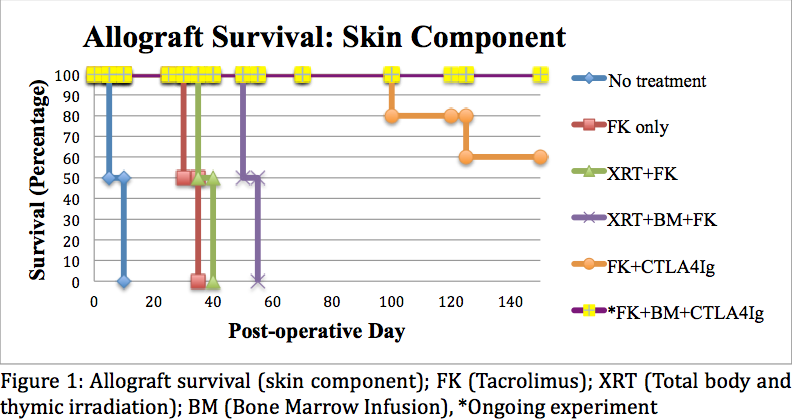
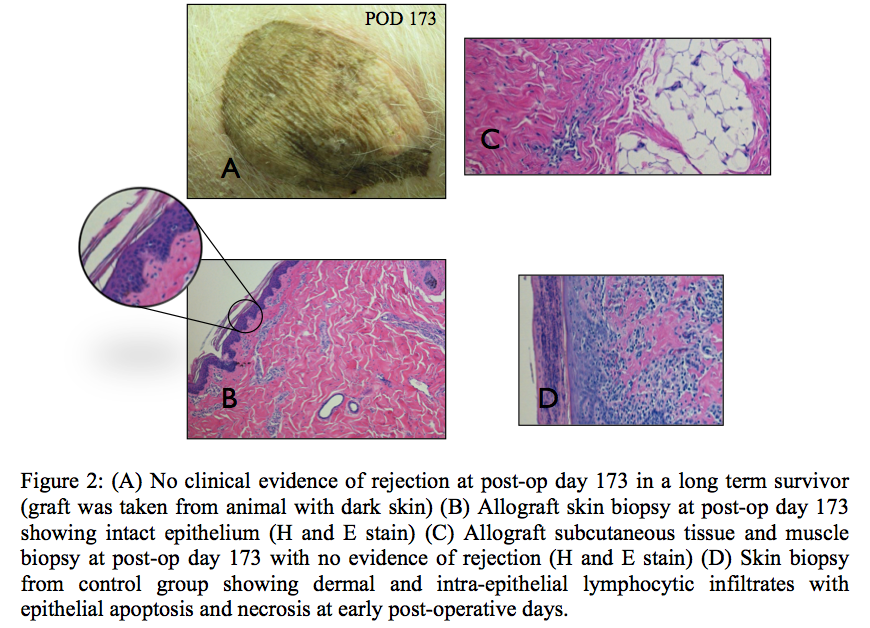
Microchimerism was established in all animals after BM cell infusion. Transplanted animals in Group 1 (Control) rejected the allograft 5 to 8 days after transplantation. Group 2 (Tacrolimus only) rejected the allograft (skin and muscle) 30-32 days after transplantation. Group 3 (Irradiation and Tacrolimus) rejected the allograft (skin and muscle) 35-37 days after transplantation. Group 4 (Irradiation, BM and Tacrolimus) rejected the skin portion of the allograft at 50, 52, and 53 days post-transplant. Remaining allograft components (muscle, bone, nerve, vessel) survived indefinitely. Group 5 (CTLA4Ig and Tacrolimus) animals demonstrated significantly prolonged muscle survival beyond 150 days post-transplant; the skin component survived past 150 days in 3 out of 5 animals (Figure 1). Skin and muscle histology in all long-term surviving animals were rejection-free (Figure 2). Group 6 (CTLA4Ig, BM and Tacrolimus) is currently in progress with rejection free survival beyond 140 days post-transplant in all animals.
CONCLUSIONS:
High-dose BM cell infusion results in stable levels of micro-chimerism. The addition of co-stimulatory blockade (CTLA4Ig) enabled us to optimize induction therapy, further reduce maintenance immunosuppression, and indefinitely prolong graft survival. Such targeted immunomodulatory protocols that combine BM cell-based strategies and biologics might facilitate immune tolerance and eliminate the need for multi-drug immunosuppression to maintain graft survival after VCA.
Back to Annual Meeting Program
|








