|
|
 |
Back to Annual Meeting Program
Novel and Translational Loading of Monocytes with Indocyanine Green for Imaging Cutaneous Inflammation
Joani M. Christensen, BA, Yongping Chen, MD, Gabriel A. Brat, MD, Kate J. Buretta, BS, Damon S. Cooney, MD, PhD, Gerald Brandacher, MD, Kristine Johnson, MD, W P Andrew Lee, MD, Xingde Li, PhD, Justin M. Sacks, MD.
Johns Hopkins University School of Medicine, Baltimore, MD, USA.
BACKGROUND: Cutaneous infection, such as a surgical site infection, can be easily mistaken for other conditions associated with edema, erythema, tenderness and warmth. Incorrect diagnosis leads to unnecessary antibiotic use, greater healthcare costs and may result in higher rates of recidivism. Currently, clinical distinction between sterile inflammation and infection relies upon interpretation of clinical parameters and microbiologic data combined with nonspecific imaging. Assessing the characteristic variations in leukocytic infiltration of sites of infection and inflammation can provide more specific information. Biopsy and radioactive cell tagging are the available methods for assessing leukocytic infiltration. A less invasive, non-radioactive mechanism for accurately tracking inflammatory cells would be of great clinical utility. Homing of systemically administered monocytes tagged using the only FDA-approved near infrared dye, indocyanine green (ICG), may be assessed using clinically-applicable laser angiography systems in order to investigate cutaneous and subcutaneous inflammatory processes.
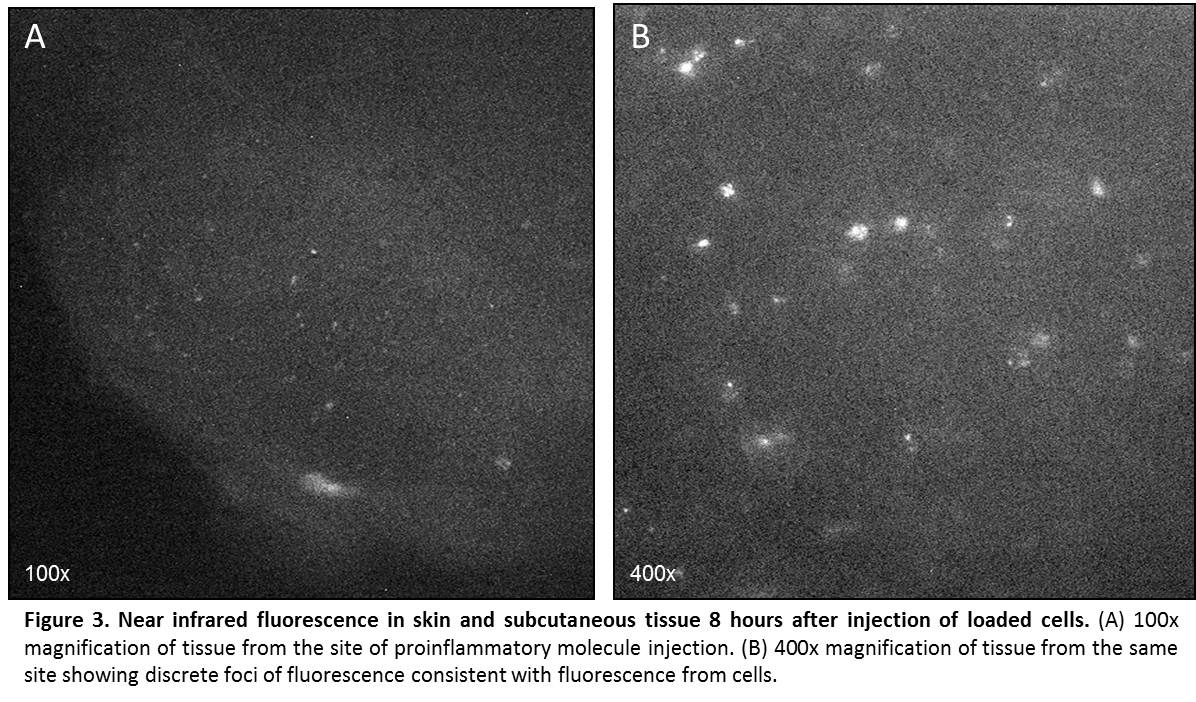
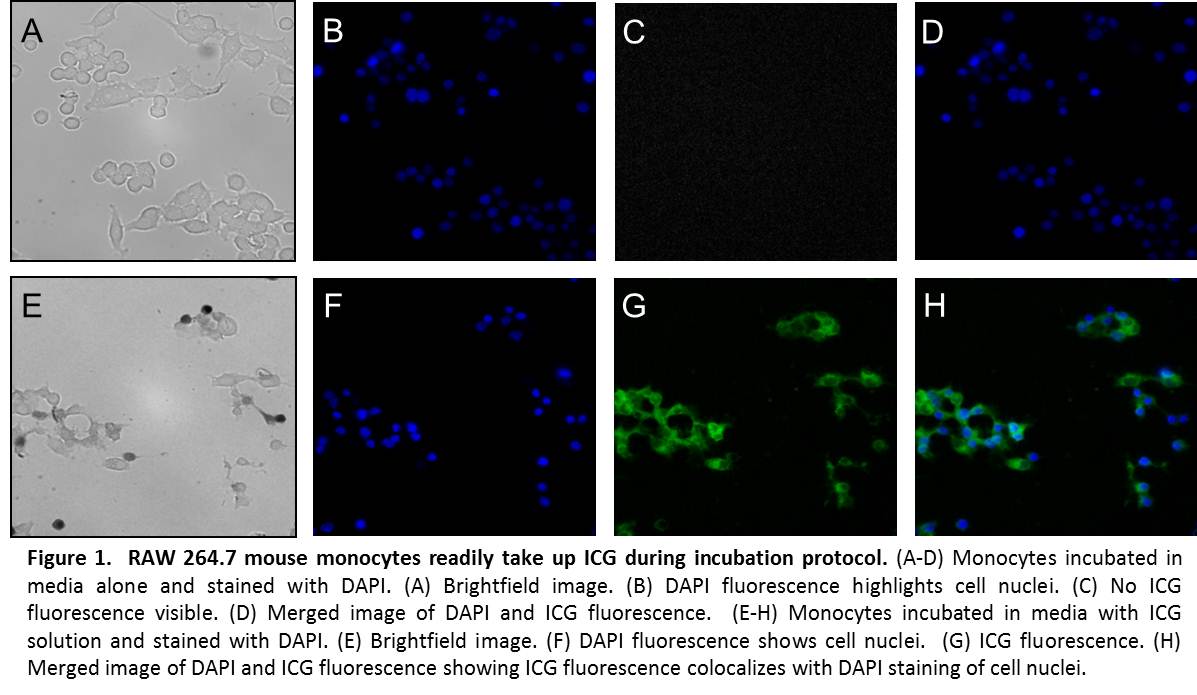
METHODS: The cellular loading protocol was optimized for the carrier solution, ICG concentration, incubation time, and washing procedure. RAW 264.7 mouse monocytes were coincubated with ICG solution and fluorescence confirmed microscopically. Cell viability was assessed. Homing ability of ICG-loaded cells was assessed in vitro using a microplate chemotaxis assay. Systemic injection of 2 million labeled cells in suspension was made into mice with induced sterile focal inflammation or infection of the lateral hind limb. Whole animal, non-invasive, near infrared imaging was completed using a FDA-approved, commercially available near infrared laser angiography system. Near infrared fluorescence in the skin and subcutaneous tissue was confirmed microscopically in fresh frozen tissue from the area of the injection.
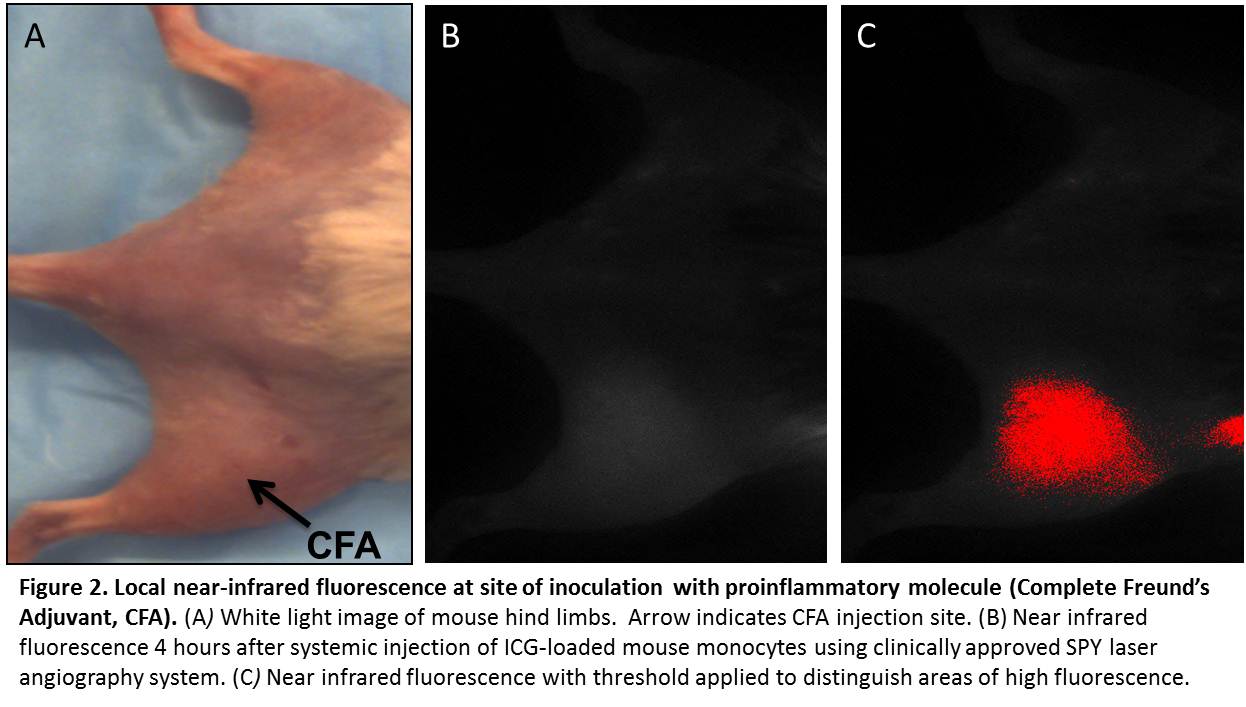
RESULTS: Monocytes readily take up ICG in culture (Fig. 1). Cell viability by trypan blue exclusion remained >50% after loading. Loaded cells displayed high near-infrared fluorescence intensities (165 a.u.) compared to unloaded cells (12 a.u.) (p < 0.01). Ability of ICG-loaded cells to chemotax in response to monocyte chemotactic protein-1 remained above baseline (p < 0.01), with a chemotactic index of 1.76. Following systemic intravascular injection of loaded cells, distinct local fluorescence was visible at the site of inflammation. Peak fluorescence was seen 4-6 hours after injection, and levels returned to baseline by 12 hours (Fig. 2). Microscopic examination of frozen tissue from the area of injection revealed punctate areas of near infrared fluorescence, consistent with the presence of ICG-loaded cells (Fig. 3).
CONCLUSION: Development of a technique to rapidly, non-invasively image inflammation without radiation can have applications in the diagnosis of many pathological conditions such as surgical site infections.
Back to Annual Meeting Program
|









