|
Back to Annual Meeting
Analysis Of specc1lb Expression And Function In Zebrafish Model Of Oblique (Tessier) Facial Cleft
Lisa Gfrerer, MD PhD1, Valeriy Shubinets, MD1, Cynthia Morton, PhD2, Richard Maas, MD PhD1, Eric C. Liao, MD PhD1.
1Massachusetts General Hospital, Boston, MA, USA, 2Brigham and Womens Hospital, Boston, MA, USA.
BACKGROUND:
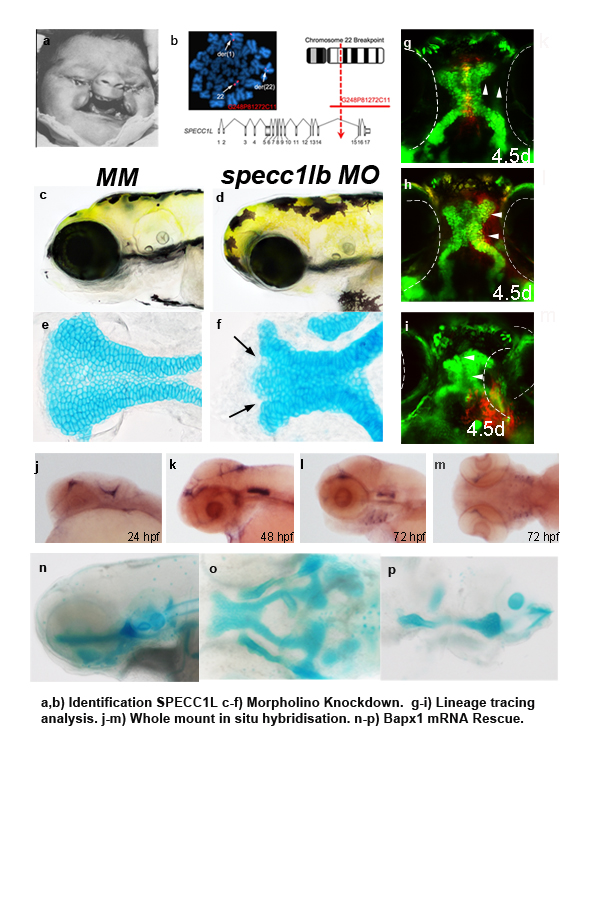
The genetic basis of oblique facial (Tessier) cleft has been unknown until the identification of SPECC1L from human translocation analysis. SPECC1L encodes a novel protein with coiled-coiled domains, and has been shown to co-localize with actin and tubulin, depletion of which results in defects in cell adhesion in culture. However, the function of SPECC1L in pharyngeal morphogenesis remains unknown. We carried out detailed analysis of SPECC1L in zebrafish (specc1lb) in order to elucidate its spatiotemporal expression and function in craniofacial morphogenesis. Moreover, we interrogated specc1lb function in the context of signaling pathway that regulates convergence and extension morphogenesis, as we hypothesize specc1lb is a structural protein that acts to integrate major signaling cascades with transcriptional control.
METHODS:
Spatiotemporal expression analysis of zebrafish specc1lb was performed across embryonic developmental stages using whole-mount in situ hybridization (WISH). Functional analysis was performed via morpholino-mediated knockdown. Fate mapping was carried out in morphants using the sox10:kaede transgenic line, in which kaede expression is transcriptionally restricted to the neural crest lineage.
RESULTS:
WISH revealed that specc1lb has dynamic embryonic expression in pharyngeal neural crest derived mesenchyme. Morpholino knockdown of specc1lb resulted in bilateral clefts between the median and lateral parts of the anterior palate, analogous to bilateral facial cleft in mammals. Lineage tracing analysis revealed that the neural crest cells that normally contribute to the frontonasal prominence failed to fuse with the maxillary prominence, providing morphogenetic basis for the facial cleft phenotype. Further, analysis of neural crest markers revealed that the expression of Wnt pathways genes frzb and bapx1 genes were down-regulated in specc1lb morphants, placing specc1lb to function in signal integration between Wnt pathway and transcription factor bapx1. Further, co-injection of bapx1 mRNA into specc1lb morphants rescued lower jaw elements, confirming that bapx1 acts downstream of specc1lb in jaw specification.
CONCLUSION:
Discovery of SPECC1L mutation as the genetic basis of oblique facial cleft has provided novel insight into the understanding of facial morphogenesis. Genetic and cellular analysis presented here demonstrate the expression and function of specc1lb to integrate Wnt signaling with bapx1 transcriptional activation. We show that this specc1lb is required for fusion of the frontonasal prominence with the paired maxillary prominences, validating its role in facial morphogenesis. , Ongoing work is aimed to elucidate the biochemical relationship between specc1lb and its interacting proteins in the context of cranial neural crest migration and morphogenesis.
Back to Annual Meeting
|







