Back to 2014 Annual Meeting Abstracts
Tamoxifen (selective estrogen-receptor modulators ) and aromatase inhibitors as potential perioperative thrombotic risk factors in free flap breast reconstruction
Michael N. Mirzabeigi, MD, Jonas A. Nelson, MD, Stephen J. Kovach, MD, Liza C. Wu, MD, Joseph M. Serletti, MD, Suhail Kanchwala, MD.
University of Pennsylvania, Philadelphia, PA, USA.
BACKGROUND: Estrogen hormone therapy (HT) utilizing selective estrogen-receptor modulators (SERMs) and aromatase inhibitors (AIs) has become ubiquitous in the treatment of breast cancer. HT is; however, a well-established thromboembolic risk factor. Given the theoretical concern for increased microvascular thromboses, recently published level III data suggests that preoperative Tamoxifen usage nearly doubles flap-related complications. Subsequent to this data, the evidence-based proposal has been to hold Tamoxifen for at least 28 days prior to reconstruction. The purpose of this study is to two-fold 1) further evaluate Tamoxifen as a potential thrombotic risk factor 2) evaluate AIs as a potential novel risk factor.
METHODS: Patients were identified via a prospectively maintained database of abdominally-based free flaps performed from January 2008 - July 2012. As per institutional protocol, patients were instructed to cease Tamoxifen two weeks prior to surgery. Patients were not advised to cease their AI regimen. Univariate statistical analyses included Fisher’s exact test and the Mann-Whitney U test. A value of p<0.05 denoted statistical significance.
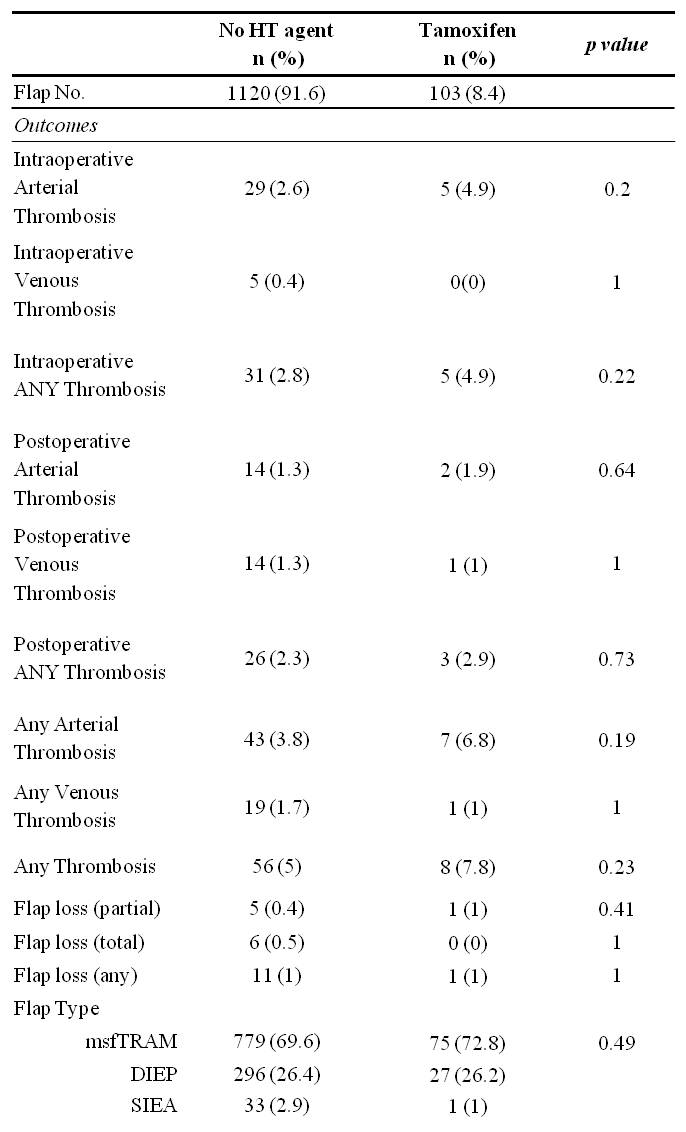
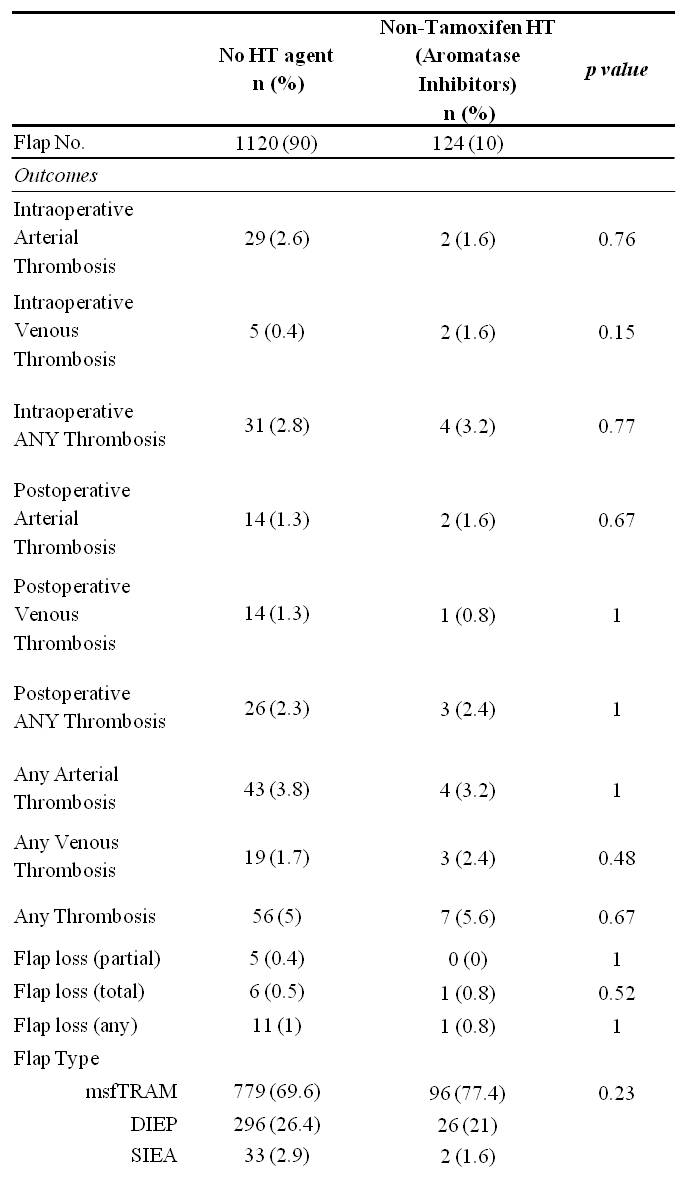
RESULTS: 1,347 flaps were performed on 851 patients. Those receiving HT prior to reconstruction had significantly higher rates of preoperative radiation, preoperative chemotherapy, and delayed reconstruction. Thrombotic complications and flap failure were analyzed per HT regimen. There were no statistically significant differences in thrombotic complications or flap failure in comparing those that did not receive preoperative Tamoxifen versus those that did receive Tamoxifen (Table 1), nor were there significant differences specific to those receiving Aromatase inhibitors (Table 2). A post-hoc power analysis was performed with the supposition that HT exposure results in a two-fold increase in complication rate. The study power was found to be 0.863.
CONCLUSIONS: Tamoxifen may have been previously overestimated as a microvascular thrombotic risk factor. At a minimum, this data suggests that withholding Tamoxifen for two weeks prior to surgery can mitigate thrombotic risk. Given the 14 day half-life of Tamoxifen’s active metabolite, Tamoxifen may be of minimal microvascular thrombosis risk altogether. AIs were not found to increase thrombotic complications despite continued perioperative administration. Given the adequate study power (>0.800), there is a low likelihood of Type II error. We recommend withholding Tamoxifen for no longer than two weeks prior to surgery and suggest further study as an even shorter time interval may be appropriate.
Back to 2014 Annual Meeting Abstracts
|




