|
|
|
|
|
Back to 2014 Annual Meeting Abstracts
Is Vicryl mesh really a safe alternative to acellular dermis in tissue expander-based breast reconstruction?
Stacy Henderson, M.D., Brett Michelotti, M.D., Susie Sun, M.D., Cathy R. Henry, M.D., T. Shane Johnson, M.D., Dino Ravnic, D.O., Donald Mackay, M.D., John Potochny, M.D..
Penn State Milton S. Hershey Medical Center, Hershey, PA, USA.
Background: The use of an inferolateral sling as an adjunct to traditional submuscular tissue expander placement following mastectomy has proven to expedite time to final reconstruction. Studies have suggested that the use of human acellular dermal matrix as an inferolateral sling portends an increased rate of complications when compared with total submuscular tissue expander placement. Use of non-biologic material, instead of acellular dermis, has been recently introduced as a low-cost alternate with few complications. The purpose of this study was to compare the complications associated with use of Vicryl mesh versus traditional submuscular coverage in immediate tissue expander-based breast reconstruction.
Methods: A retrospective review was conducted of all patients who underwent immediate tissue expander-based breast reconstruction at a single academic institution between December 2012 and December 2013. This case-control study consisted of patients in whom Vicryl mesh was placed as an inferolateral sling and patients whose breast tissue expanders were placed with total muscular coverage. Patients who underwent direct to implant reconstruction, tissue expander placement under a pedicled or free muscle flap, or delayed tissue expander placement were excluded. We then compared perioperative variables and incidence of complications between groups using both univariate and multivariate analyses.
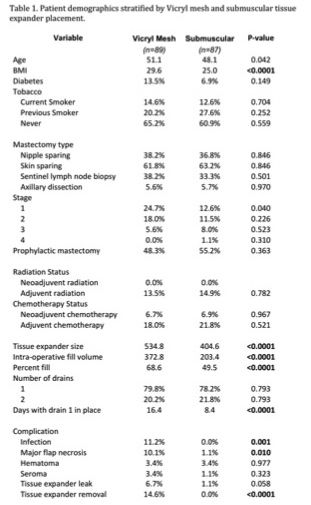
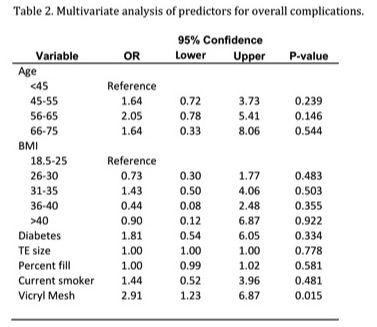
Results: A total of 176 breasts met inclusion criteria: 51% (n = 89) with Vicryl mesh and 49% (n = 87) with no Vicryl mesh. When compared to the submuscular cohort, the Vicryl mesh group had a significantly larger tissue expander size, intraoperative fill volume, and percentage of initial tissue expander fill (p = <0.0001). The Vicryl mesh group also had a higher rate of infection requiring intravenous antibiotics (11.2% vs. 0%; p = 0.001), major flap necrosis (10.1% vs. 1.0%, p = 0.01) and tissue expander explantation due to infection or necrosis (14.6% vs. 0%, p = <0.0001). On multivariate analysis, Vicryl mesh was associated with 2.91 times higher risk for overall complications when compared with traditional submuscular tissue expander placement (p = 0.015). Diabetes and current tobacco use were independent predictors of infection.
Conclusions: Vicryl mesh provides an alternative to acellular dermal matrix for use as an inferolateral sling in tissue expander-based reconstruction. It allows increased tissue expander size and intraoperative fill volume, which may be associated with decreased time to final reconstruction. However, this study reveals that its use is associated with a significantly increased risk of complications including infection, flap necrosis, and tissue expander removal.
Back to 2014 Annual Meeting Abstracts
|
|




