|
|
|
|
|
Back to 2015 Annual Meeting
An Electrospun Fiber-Hydrogel Composite with Interfacial Bonding for Soft Tissue Regeneration in vivo
Georgia C. Yalanis, BS, MSc1, Russ Martin, B.S.2, Sashank Reddy, M.D.1, Jisuk Choi2, Denver M. Lough, M.D.1, Hai-Quan Mao, Ph.D.2, Justin M. Sacks, M.D.1.
1Johns Hopkins University, Department of Plastic and Reconstructive Surgery, Baltimore, MD, USA, 2Johns Hopkins University, Whiting School of Engineering, Baltimore, MD, USA.
Background: Reconstructive procedures for acquired surgical defects using autologous or synthetic tissue can lead to morbidity both at the donor site or infection, device failure, and fibrosis. An alternative approach using the body's regenerative capacity and an appropriate synthetic scaffold would allow restoration of form permitting regeneration of native tissue. We developed a novel fiber-hydrogel composite using FDA-approved materials with optimal biomechanical properties, porosity, crosslinking density, and interfacial bonding to promote soft tissue regeneration in vivo.
Methods: Fiber-hydrogel composite preparation. Electrospun poly-ε-caprolactone (PCL) fibers grafted with poly-acrylic acid (PAA) and surface-modified with maleimide groups were ground using a cryo-milling system to uniformly disperse fibers inside a hyaluronic acid (HA) hydrogel. Control was a hydrogel with similar mechanical properties. Mechanical properties were measured by compressive and shear storage modulus. GFP labeled adipose-derived stem cell (ASC) spheroids were seeded inside the composite evaluating ASC migration.
Biocompatibility in vivo. Composites were implanted under the inguinal fat pad of nine, 8-12 week old male Lewis rats. HA controls were implanted into 9 rats. Three sham surgeries were performed to characterize surgical site inflammation. Composites and HA controls were explanted en bloc in groups of 3 animals at 1, 2, and 4 week intervals. These samples were fixed and stained using H&E and Masson Trichrome. Naïve fat pads harvested at 1 week after sham surgeries were also explanted, fixed, and stained.
Results: Morphology of the composite mimicked extracellular matrix composed of collagen and elastin with a high porosity (Figure 1, A and B). Migrated ASCs were increased in the composite compared to control (Figure 1, C and D). We postulated composite nanofibers become a guide similar to the native cytoskeleton permitting cellular migration in vitro.
Prior to explant in vivo, the composite revealed no evidence of infection or fibrosis, demonstrating incorporation into native fat. Histology after 4 weeks demonstrated no inflammatory response above a surgical site response or cellular atypia. H&E and Masson Trichrome staining demonstrated septation and cellular infiltration by the native fat through the composite, capillary formation around the perimeter, and regeneration of the glandular as well as adipocyte portions of native fat. Control lacked cellular infiltration and differentiation, revealed inflammation above a surgical site response, foreign body reaction, and fibrosis (Figure 2).
Conclusion: We developed a novel fiber-hydrogel composite with optimal porosity, crosslinking density, and a high ratio of interfacial bonding that promotes cellular migration in vitro. Our composite maintains 3-dimensional structure in vivo while promoting cellular infiltration, differentiation, angiogenesis, adipogenesis, and regeneration of native soft tissue without a foreign body response or fibrosis. Use of our novel composite material has significant implications for reconstituting soft tissue defects in vivo without the negative sequelae associated with autologous reconstruction or implant-based devices. 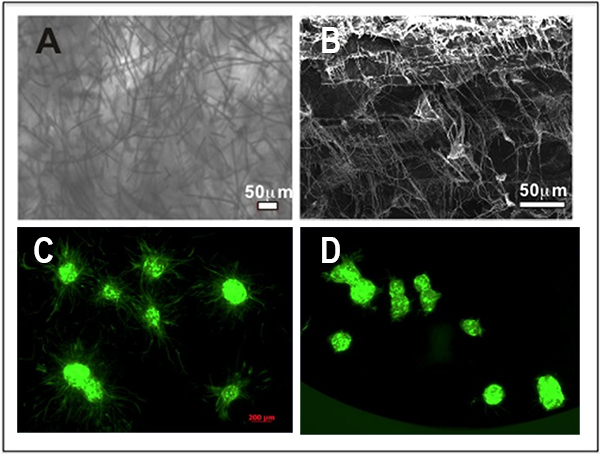 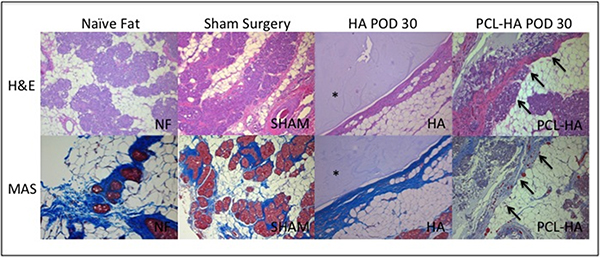
Back to 2015 Annual Meeting
|
|




