|
|
|
|
|
Back to 2016 Annual Meeting
Immunomodulation in Vascularized Composite Allotransplantation – Preliminary Results in a Non-Human Primate Model with Tocilizumab
Zhi Yang Ng, MD, Alexandre G. Lellouch, MD, Matthew W. Defazio, BS, Zachary W. Heroux, BS, Jigesh A. Shah, DO, Josef M. Kurtz, PhD, Curtis L. Cetrulo, Jr., MD FACS FAAP.
Massachusetts General Hospital, Boston, MA, USA.
BACKGROUND: Long-term tolerance of transplanted kidneys has been demonstrated clinically through the mixed chimerism strategy, which involves bone marrow cell (BMC) infusion and nonmyeloablative conditioning. However, application in face and hand transplantation (i.e. vascularized composite allografts, VCAs) without preconditioning has been unsuccessful thus far. We report prolonged immunosuppression-free VCA survival using a clinically-relevant protocol in a pre-clinical large animal model. Briefly, VCA is first performed followed by BMC infusion at a later date. This approach is believed to abrogate the peri-operative inflammatory mileu and promote engraftment of BMCs.
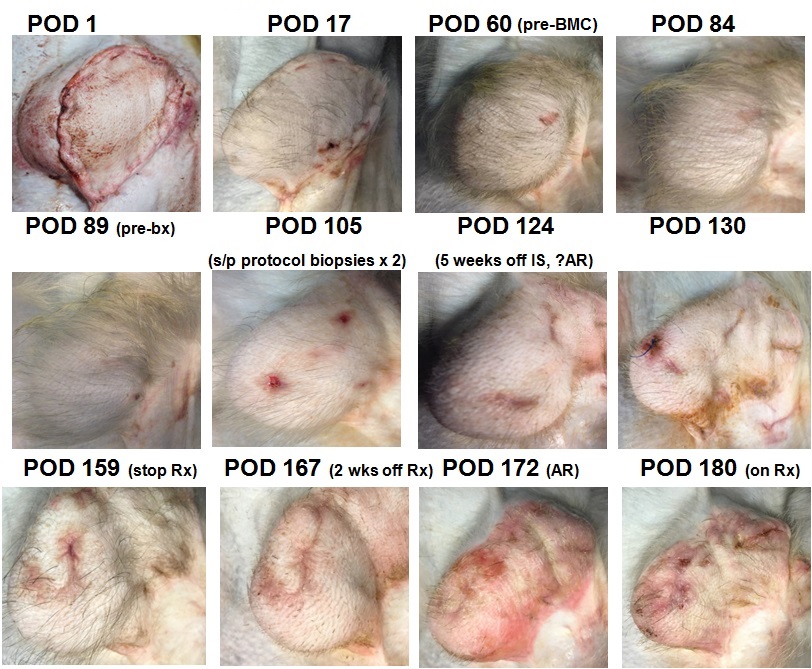
METHODS: An osteomyocutaneous facial VCA was transplanted heterotopically into a MHC-mismatched non-human primate (NHP) following induction therapy with anti-thymocyte globulin and maintained post-operatively with FK506, methylprednisolone, and MMF. Prior to infusion of cryopreserved donor BMCs on POD 60, the NHP underwent nonmyeloablative conditioning consisting of total body and thymic irradiation, and further lymphocyte depletion; additional immunotherapy with 5c8, anti-IL6 mAb, and cyclosporine-A was instituted before complete withdrawal of all immunosuppression from POD 90 onwards. VCA was assessed by serial clinical examination and histopathology. Chimerism was monitored by flow cytometry; in vitro immunologic responses were measured with CFSE mixed lymphocyte reaction (MLR) and serum allo-antibody assays.
RESULTS: The NHP remained rejection-free both clinically and on histopathology prior to delayed BMC infusion. At POD 126 (5 weeks off of all immunosuppression, 9 weeks after BMC infusion), an episode of suspected acute rejection developed in the VCA. Biopsy results were equivocal and clinical resolution was achieved following steroid bolus and a tapering course of FK506 (to stop at POD 158). Follow-up biopsy of the VCA at POD 143 showed no evidence of rejection. However, at POD 172 (further 2 weeks off immunosuppression), clinical rejection developed requiring re-instatement of steroid and FK506 treatment (Figure 1). In vitro assays demonstrate decreased anti-donor responses after BMC infusion and generalized hyporesponsiveness a week prior to rejection; no antibody formation was detected at all time points. Evidence suggestive of mixed chimerism was only detected transiently at 6 weeks after BMC infusion. To date (POD 185, 17 weeks post-BMC infusion), no systemic sequelae of GvHD, PTLD or evidence suggestive of chronic rejection have been observed.
CONCLUSIONS: Unlike kidney allografts in which transient mixed chimerism was sufficient in inducing transplantation tolerance, our results suggest otherwise. Continued follow-up is required to determine if reinstatement of immunosuppressive therapy will be able to salvage the VCA and has direct relevance to clinical trials on tolerance induction in VCA. Further studies in our laboratory are focused on achieving stable engraftment of BMCs for the generation of durable mixed chimerism and the attendant tolerance of VCA.
Back to 2016 Annual Meeting
|
|
