Back to 2016 Annual Meeting
Breast tissue expanders and MRI: Defining surgeons’ opinions, clinical outcomes, and optimal parameters for safety
Peter W. Henderson, MD MBA1, Nanda D. Thimmappa, MD1, Martin R. Prince, MD PhD1, Christine H. Rohde, MD MPH2.
1Weill Cornell Medical College, New York, NY, USA, 2Columbia University Medical Center, New York, NY, USA.
BACKGROUND: Manufacturers’ claims that internal (ferromagnetic) port-containing breast tissue expanders (FPCBTE) are contraindicated in magnetic resonance imaging (MRI) surprisingly lack direct experimental basis, and may unwittingly negatively impact patient care. This study sought to define surgeons’ opinions on the compatibility of MRI and FPCBTE, determine if they can be safely combined, and identify modifiable variables that can maximize safety.
METHODS: First, ASPS members were given a validated, web-based survey of their opinions on the compatibility of FPCBTE and MRI. Second, a review of patients with FPCBTE who had undergone 1.5 Tesla (T) MRI has been performed. Finally, ex-vivo study of FPCBTE from each FDA-approved manufacturer was undertaken in different MRI settings.
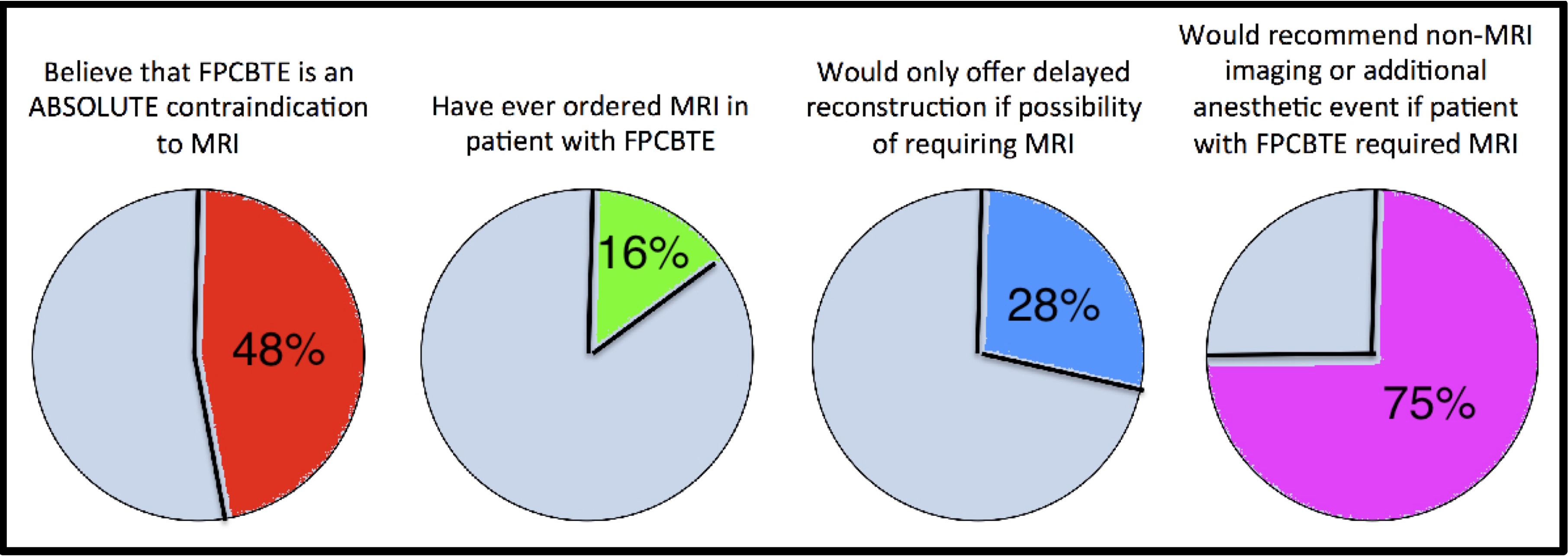
RESULTS: Most survey respondents believe FPCBTE and MRI should not be combined, and act accordingly (Figure). The clinical series found no complications among 71 women with FPCBTE who underwent 1.5T MRI. The ex-vivo study showed that Natrelle devices had the least torque and smallest deflection angles, and that unshielded 1.5T MRI exerted the least ferromagnetic effect.
CONCLUSIONS: Our study confirms that the compatibility of FPCBTE and MRI is largely misunderstood, it is possible for MRI to be safely performed with FPCBTE in place, and, in an experimental setting, Natrelle devices in unshielded 1.5T MRI were least affected by the magnetic force. This study defines a widespread misunderstanding that has implications for a generation of women, and works towards a better understanding of the variables that can be modified into order to allow women to undergo the safest, highest quality breast reconstruction.
Back to 2016 Annual Meeting
|
