|
|
|
|
|
Back to 2016 Annual Meeting
The Accuracy of Conflicts-of-Interest Disclosures Reported by Plastic Surgeons & Industry: A New Era of Transparency?
Joseph Lopez, MD MBA1, Gabriel Siegel, BA1, Taylor Purvis, BA1, Javaneh Jabbari, BA1, Rizwan Ahmed, MD2, Jacqueline Milton, PhD3, Anthony P. Tufaro, MD DDS1, James W. May, Jr., MD4, Amir H. Dorafshar, MBBS1.
1Johns Hopkins Hospital, Baltimore, MD, USA, 2Duke University Hospital, Durham, NC, USA, 3Boston University School of Public Health, Boston, MA, USA, 4Massachusetts General Hospital, Boston, MA, USA.
BACKGROUND: Recently, the public reporting of payments made to physician by biomedical companies, was made mandatory under the Physician Payment Sunshine Act (PPSA). This new policy now provides the opportunity to assess the accuracy of physicians’ conflicts of interest (COI) disclosures in scientific journals. The purpose of this study was to analyze both the accuracy of self-reported COI disclosures in the plastic surgery literature and the accuracy of the publically available physician payment database, also known as the Open Physician Payments (OPP) database. We hypothesized that the majority of monetary payments reported in the OPP database would be disclosed as COI in scientific journals.
METHODS: We analyzed all scientific articles published in four plastic surgery journals from September 2013 to January 2014. The COI disclosure statement of all these articles was reviewed and the full name and affiliation of all the investigators, regardless of whether or not he/she disclosed a COI, was recorded. All investigators were searched in the OPP database to determine whether a financial transaction was reported by a biomedical company during the study period. To further examine the factors associated with disclosure of COI in the plastic surgery literature, a multivariable regression analysis was performed.
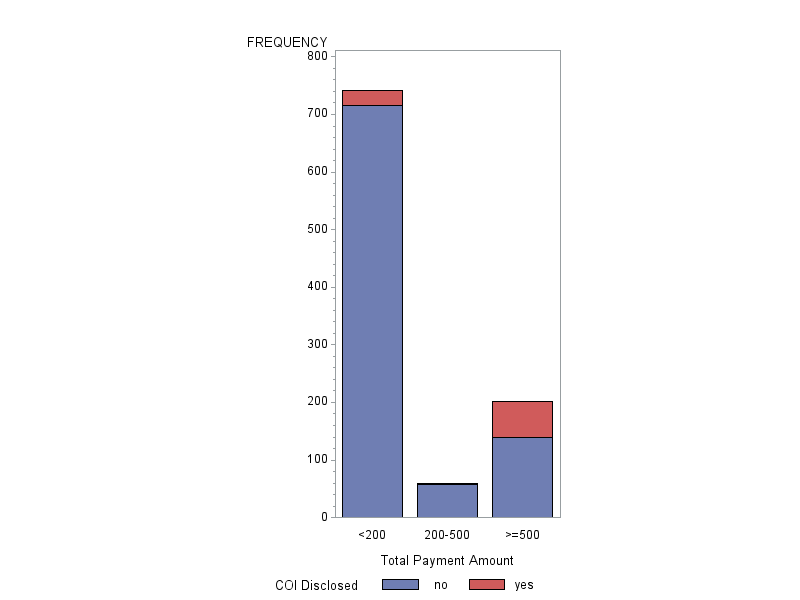
RESULTS: A total of 1002 investigators published scientific articles in the plastic surgery literature during the study period. Of these, only 90 (9%) investigators disclosed a COI. In contrast, a total of 428 (42.7%) investigators were found in the OPP database to have received monetary or in-kind payments from a biomedical device company. More specifically, 59.1% (253 out of 428) received in-kind payments, 3.7% (16 out of 428) received monetary only, and 37.2% (159 out of 428) received in-kind and monetary payments. The overall rate of disclosure was 38.9% (68 out of 175). For investigators that did not disclose a COI but received monetary payments related to the topic of the scientific article, 35.8% (125 out of 349) were directly related to the topic of the scientific article. For investigators that did disclose a COI, 24.4% (22 out of 90) were found to have discrepancies in the types of financial transactions reported in the articles and the OPP database. In the multivariate analysis, investigators that were academic (p < 0.0001), received payments that exceeded $500 in value (p < 0.0001), and published scientific articles related to the sponsoring biomedical company (p < 0.0001) were more likely to disclose COI.
CONCLUSIONS:
The incidence of industry payments reported in the OPP database greatly exceeded the rate of self-reported COI by plastic surgeons in scientific journals. Our analysis suggests that there exist major discrepancies between self-reported COI as disclosed by investigators and the industry-mandated OPP database.
Back to 2016 Annual Meeting
|
|
