A Novel Biomaterial Approach to Improve the Survival of Human Fat Graft
Brian H. Cho, MD1, Xiaowei Li, Ph.D.2, Sashank Reddy, MD1, Russell Martin, Ph.D.3, Michelle Seu, BA1, Hai-Quan Mao, Ph.D.3, Justin Sacks, MD, MBA, FACS1.
1Department of Plastic and Reconstructive Surgery, Johns Hopkins University, Baltimore, MD, USA, 2Translational Tissue Engineering Center, Johns Hopkins University, Baltimore, MD, USA, 3Department of Materials Science and Engineering, Johns Hopkins University, Baltimore, MD, USA.
BACKGROUND:Fat grafting has emerged as an important tool for soft tissue restoration and enhancement. Broad use of fat grafting has been hampered by several issues. First, poor adipocyte survival leads to variable graft take and necessitates repeat procedures to achieve a durable effect. Second, larger volume defects are difficult to restore in a predictable fashion by fat grafting alone. Third, donor site availability can be a problem in thinner patients and improper harvest can lead to contour irregularities and even bowel perforation. To address these limitations, we have developed a nanofiber-hydrogel composite scaffold to be used in combination with fat grafting. We hypothesize that our composite can retain host growth factors within the stromal vascular fraction of the fat graft to enhance its survival and integration for soft tissue reconstruction.
METHODS:We have developed a unique composite scaffold by interfacially bonding biodegradable poly(caprolactone) nanofibers with hyaluronic acid hydrogel (Fig.1). Our composite exhibits a similar fibrillar microarchitecture as the native adipose tissue. Our composite was designed to match the mechanical properties of native fat tissue with an average of shear G' of 150 Pa, while the hydrogel phase in the composite has a porosity of a G' of 80 Pa. To test the ability of this composite in supporting fat graft survival, we first isolated human adipose-derived stem cells (hASCs) from donor fat graft, then cultured hASCs in spheroid form in the composite, and assessed cell survival and migration in vitro. We subcutaneously injected hASC spheroids in the composite into Lewis rats and examined the ability of our composite to promote hASC survival, differentiation, and host-tissue integration. We also cultured freshly harvested human liposuction aspirate with this composite and characterized adipocyte survival through cell viability assays and immunohistochemistry analysis. In addition, we are assessing composite-mediated growth factor retention.
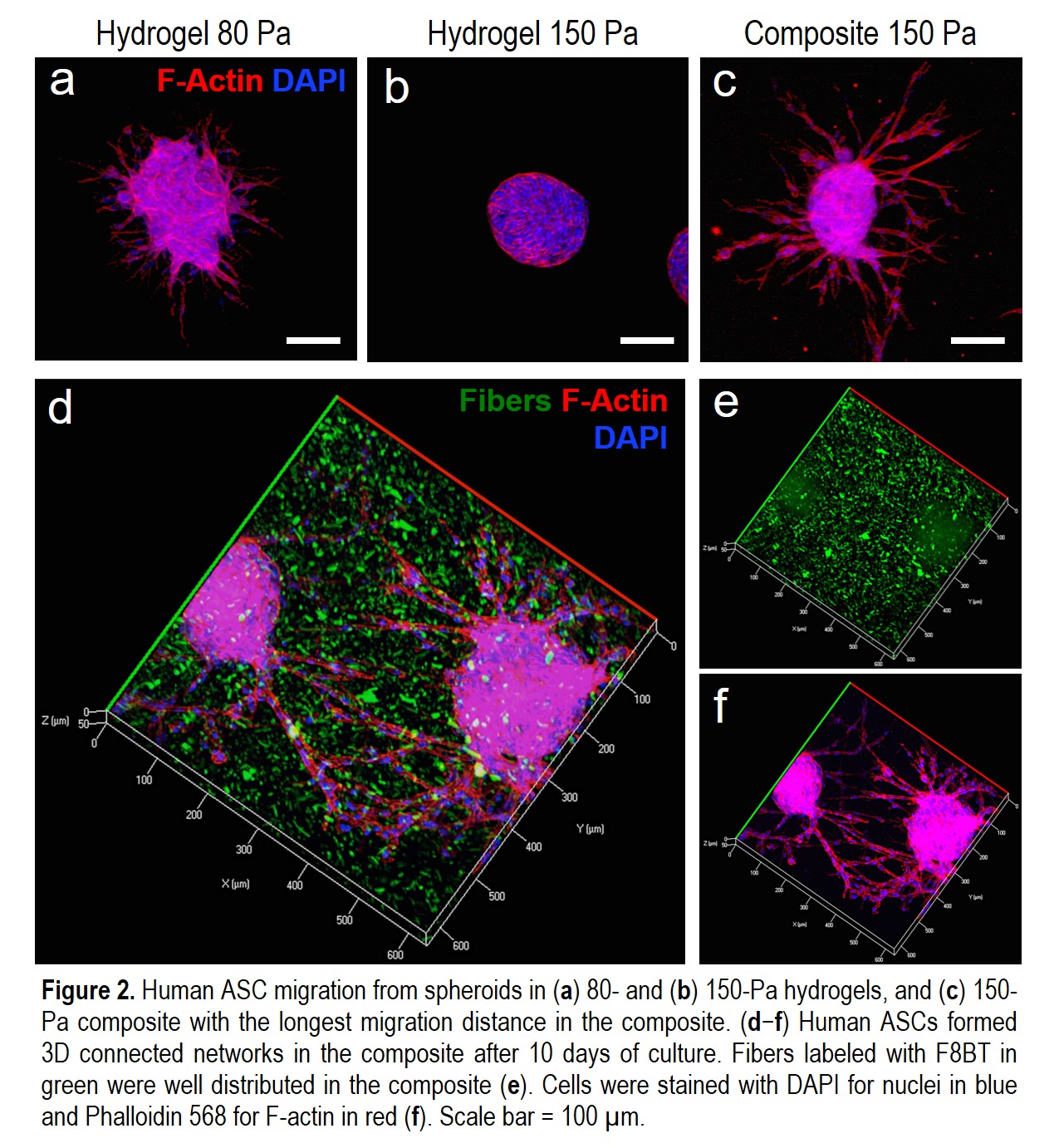
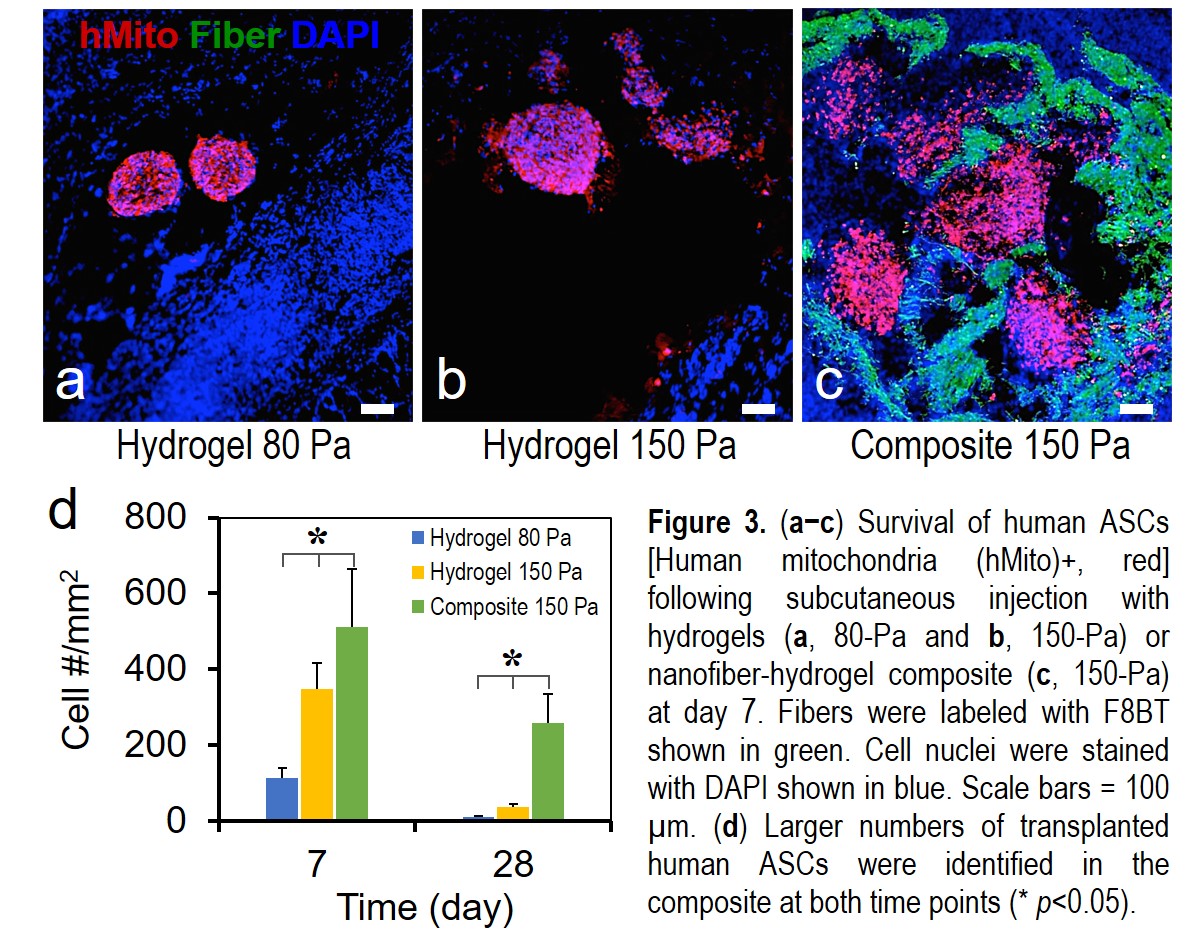
RESULTS:Human ASCs within the composite migrated the longest average distance compared to the soft (80 Pa) and medium (150 Pa) hydrogels (Fig.2a-c). Human ASCs migrated effectively in 3D and formed networks in the composite (Fig.2d-f). In the rat model, transplanted hASCs showed a higher degree of survival and spreading within our composite compared to those in the hydrogels at both day 7 and 24 (Fig.3). We are currently quantifying the ability of composite in promoting human fat graft survival and characterizing composite-mediated growth factor retention effect.
CONCLUSIONS:We have developed a unique composite to support hASC spreading and survival following transplantation. This biomaterials approach to improve fat graft survival and volume retention addresses the current limitations of fat grafting while potentially broadening its use in reconstructive and aesthetic surgery. 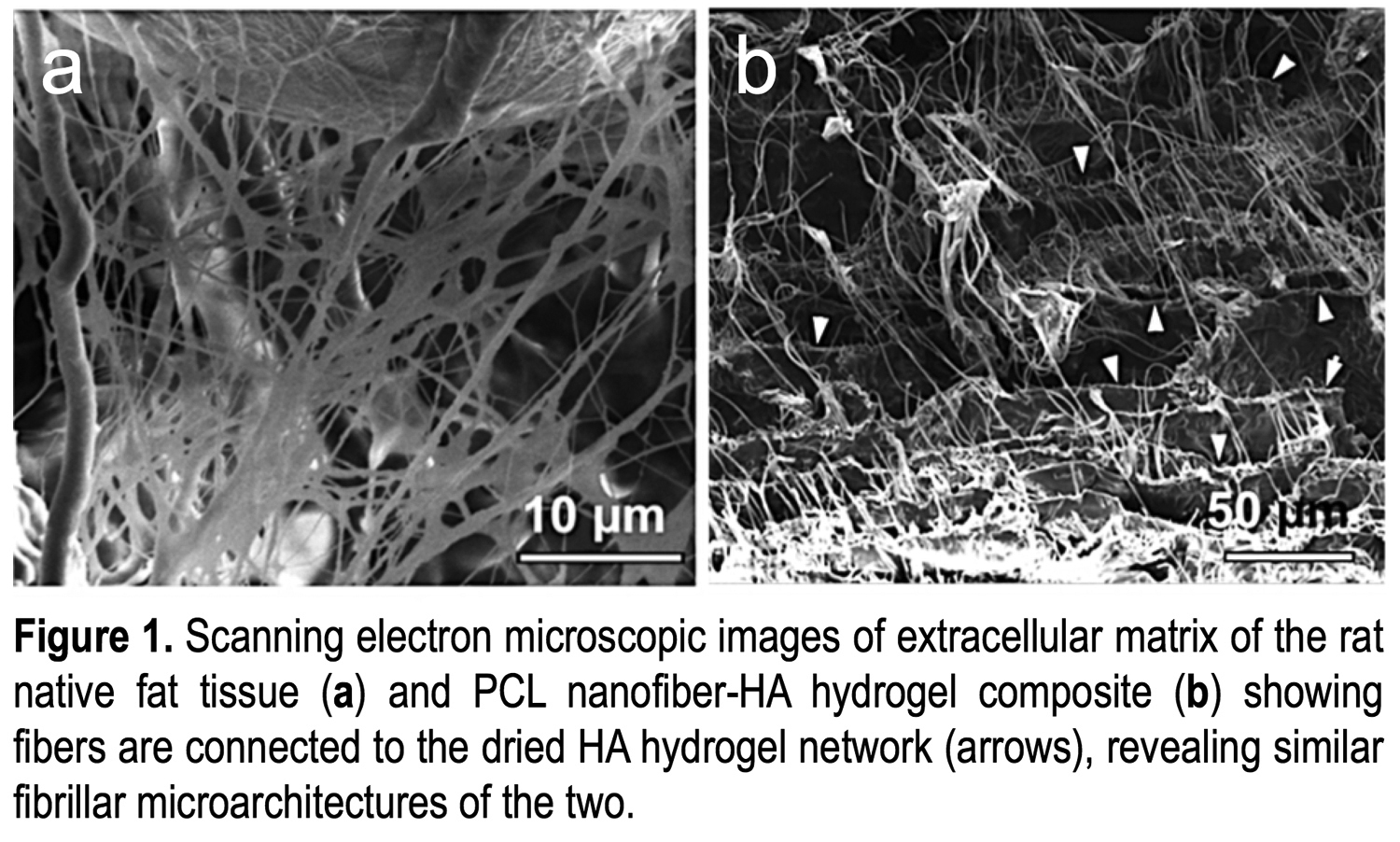
Back to 2017 Program




