Orchestrating Hierarchical Angiogenesis with Bioactive Sphingolipids
Andrew I. Abadeer, M. Eng, Julia Jin, Yoshiko Toyoda, Brandon Gold, John Morgan, PhD, Jason Spector, M.D..
Weill Cornell Medical College, NY, NJ, USA.
Background
For reconstructive surgeons, there exists a tremendous need for a product that can offer both skin coverage and host integration without the limitations of donor site morbidity. Still, the potential of current "off-the-shelf" dermal replacements such as Integra have consistently been disappointing particularly in poorly vascularized wound beds. These products fail in all but the most optimal wound beds secondary to a lack of inherent vascularity. Recently, sphingosine-1-phosphate (S1P), a bioactive sphingolipid stored in platelets and found in micromolar quantities in the plasma and nearly absent in the interstitium, has gained widespread attention for its effects on endothelial motility and vascular maturation. In fact, it is thought that a reversal of the physiologic gradient causing S1P to be present in the interstitium, such as in wound healing, is responsible for inducing reparative angiogenesis. It is further hypothesized that gradients of S1P are a major signaling component in activating homeostatically nascent angiogenic mechanisms by promoting endothelial sprouting, motility, and stability. Herein we have designed a tissue engineered scaffold that utilizes S1P to selectively amplify endothelial invasion and microvascular network formation with potentially profound implications in the clinical treatment of poorly vascularized wound beds
Methods
Endothelial cells (EC) were suspended in 3 mg/ml Type 1 collagen that was impregnated with 1uM S1P at a density of 2 million cells/ml of collagen. Within the collagen, a 1mm diameter channel was cast then seeded with ECs. Constructs were sectioned axially through the channel. Confocal, fluorescent, and light microscopy were performed to quantify endolumenal diameter, total vessel length, number of sprout endpoints, junction density, and extent of invasion.
Results
In constructs treated with S1P, axial sections demonstrated robust cellular invasion into the collagen bulk originating from the "macrovascular" conduit with sprouts branching and anastomosing into an intricate microvascular plexus. Lumen diameters of tubules were similar in caliber to deep dermal arteriolar vessels. Constructs exhibited an average endothelial sprout density of 37±8 sprouts/mm2 with an average depth of invasion of 129±58 um. Constructs treated with S1P also had, on average, 10 mm greater vessel length, 170% increase in junction density, and 125% increase in total number of endpoints (p=0.0003, p<0.001, p=0.0004). Conversely, constructs without S1P exhibited confluent superficial growth but no invasion into the collagen matrix whatsoever regardless of VEGF, FGF, or serum supplementation.
Conclusions
As current tissue engineered dermal replacement products remain constrained by an inadequate induction of endothelial invasion, we have herein fabricated a regenerative template that selectively amplifies endothelial invasion and tubulogenesis to create a hierarchical, biomimetic vascular network. Such a template holds tremendous promise for clinical translation in the coverage and healing of challenging wounds.
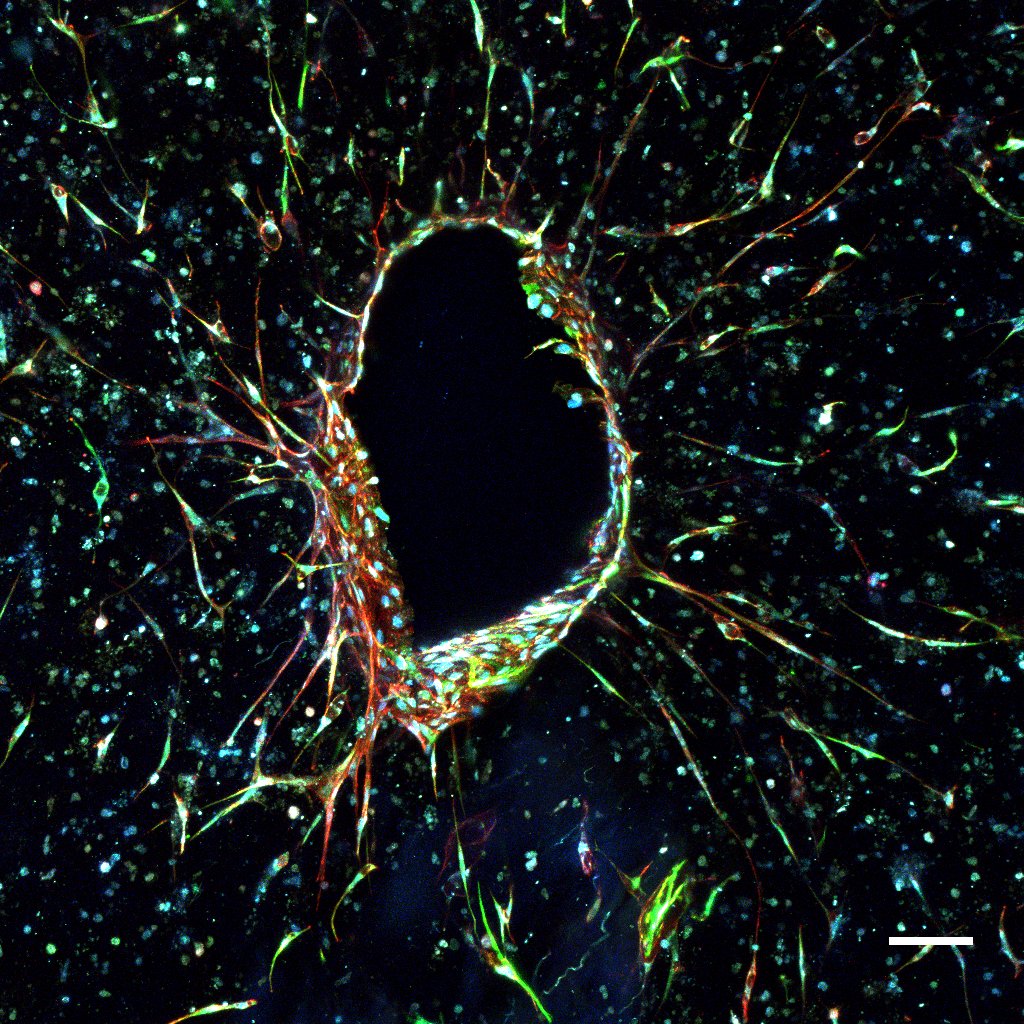
Figure 1.S1P Treated Construct Demonstrating Endothelial Outgrowth from a Central Conduit. 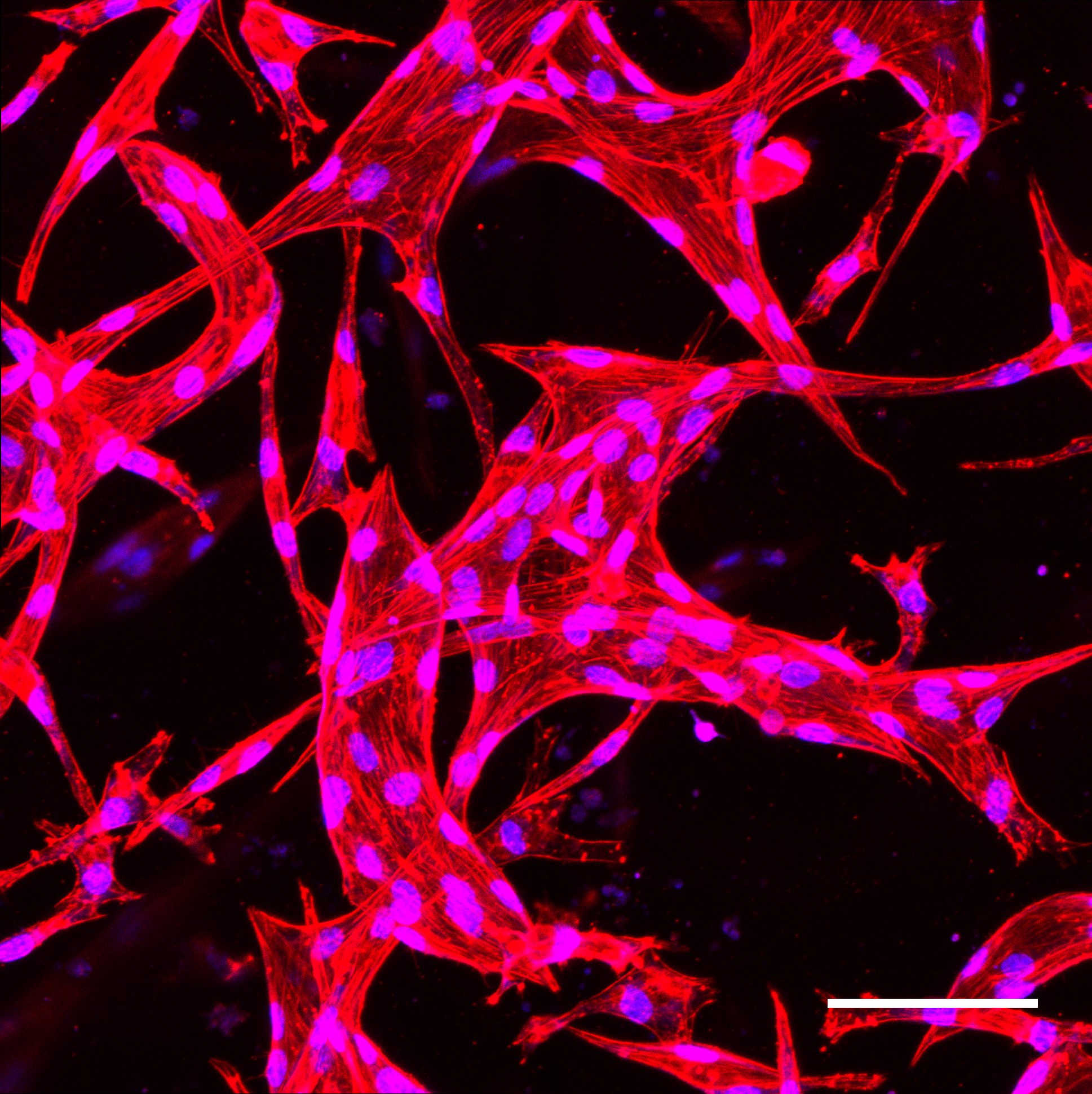
Figure 2.Endothelial Plexus. Scale Bar=100um
Back to 2017 Program




