A novel nanofiber hydrogel-based drug delivery system for sustained release of IGF-1 nanoparticles to augment functional outcomes after delayed nerve repair
Karim A. Sarhane, MD, MSc, Chenhu Qiu, BS, Benjamin Slavin, BS, Nicholas Hricz, BS, Philip Hanwright, MD, Nicholas von Guionneau, MD, Ruifa Mi, MD, PhD, Ahmet Höke, MD, PhD, Hai-Quan Mao, PhD, Sami Tuffaha, MD.
Johns Hopkins University, Baltimore, MD, USA.
BACKGROUND:Despite extensive research efforts, no therapeutic agents are clinically available for the treatment of peripheral nerve injuries. Insulin-like growth factor 1 (IGF-1) is an ideal therapeutic candidate as it can accelerate axonal regeneration and also minimize the deleterious effects of prolonged denervation on muscle and Schwann cells. However, given its short half-life, a practical delivery system is needed to stabilize the protein and provide sustained release to target tissues. Using a novel encapsulation method, we demonstrated sustained release of bioactive IGF-1 from nanoparticles, in vitro, and improved nerve regeneration and functional recovery, in vivo. Here we describe the fabrication, optimization, and characterization of a nanofiber hydrogel composite (NHC) carrier system to maintain IGF-1 nanoparticles at target tissue sites for the duration of drug release and avoid frequent re-dosing. METHODS: An injectable NHC with PCL nanofibers covalently bonded to hyaluronic acid was fabricated by electrospinning. We combined IGF-1 nanoparticles with various NHC formulation and measured IGF-1 release kinetics and bioactivity in vitro and in vivo. NHC biocompatibility, biointegration, and immune response were characterized. Finally, we used a rat model in which chronic denervation is induced prior to nerve repair in order to test the efficacy of the optimized IGF-1 nanoparticle-NCH delivery. RESULTS: The NHC architecture was found to mimic ECM fat (Figures 1A and 1B). It exhibited favorable biocompatibility with minimal inflammatory response 25 days post injection (Figures 1C, 1D, 1E, 1F). The composite system polarized the invading macrophages into an anti-inflammatory and pro-regenerative M2 phenotype (Figure 1G, 1H, 1I). As a carrier for IGF-1 nanoparticles, NHC conferred superior IGF-1 release kinetics, in vitro and in vivo, in comparison to fibrin gel and saline (Figure 1J). The optimized IGF-1 nanoparticle-NHC system was effective in reducing muscle atrophy and sustaining Schwann cell proliferation in the setting of prolonged denervation. CONCLUSIONS: We introduce a novel drug delivery system in which IGF-1 nanoparticles are combined with a nanofiber hydrogel carrier to provide sustained local concentrations of bioactive IGF-1within target nerve and muscle. This therapeutic approach has the potential to improve functional outcomes via enhanced axonal regeneration and maintenance of denervated muscle and Schwann cells. IGF-1 and the polymer components of the engineered delivery system are currently used in FDA-approved formulations and devices, which will facilitate clearance of regulatory hurdles. 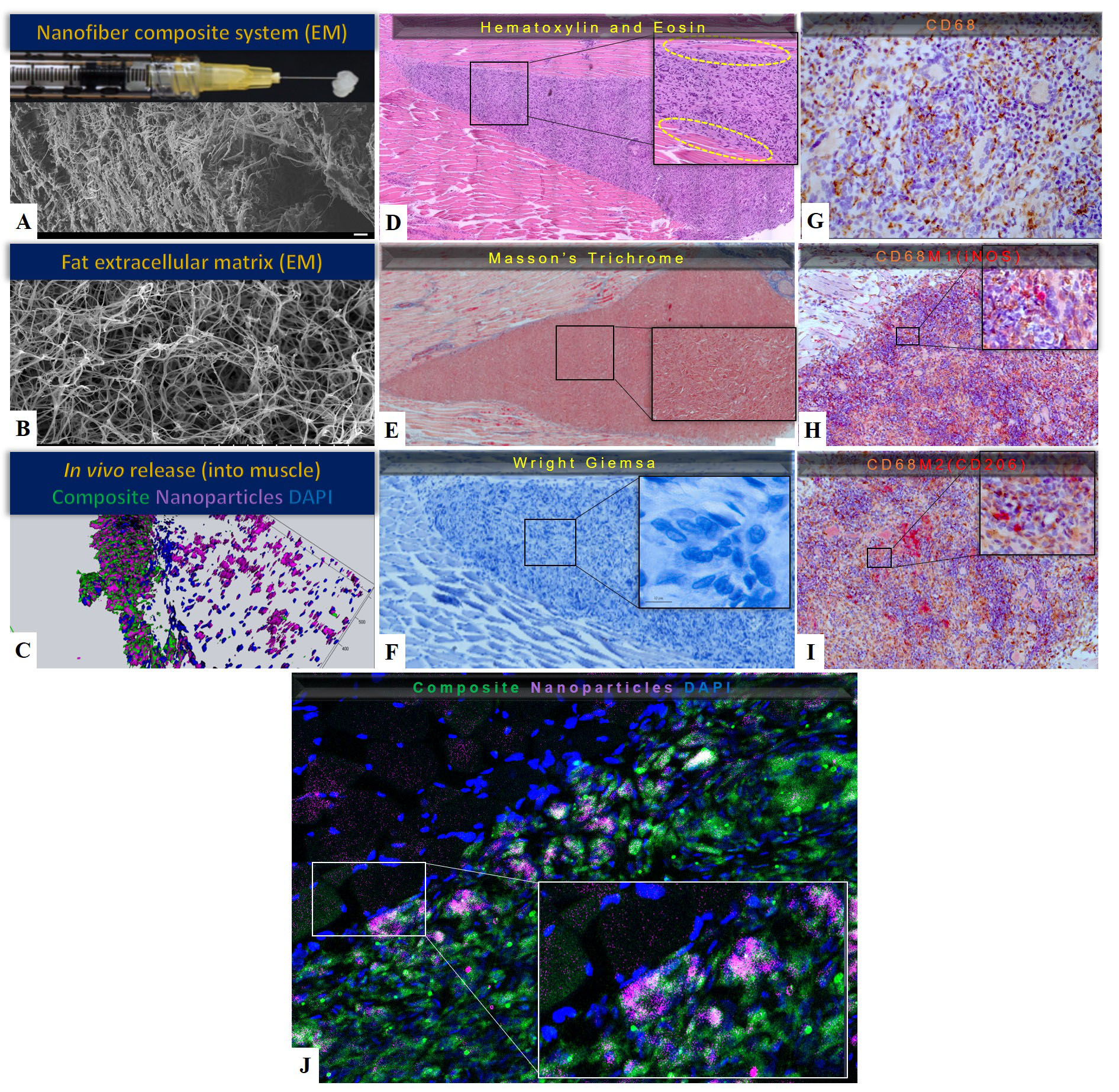
Back to 2019 Abstracts
