Characterizing the Angiogenic Capacity of Breast Cancer Cells in a High Fidelity Patient-Derived, Tissue Engineered Biomimetic Platform
Ishani D. Premaratne, BA1, Andrew J. Miller, BA1, Runlei Zhao, MD1, Daniel O. Lara, BA1, Matthew A. Wright, BA2, Arash Samadi, BA1, Ryan Bender, BA3, Cosima S. Boettner4, Kristy A. Brown, PhD1, Jason A. Spector, MD5.
1Weill Cornell Medicine, New York, NY, USA, 2Columbia University Vagelos College of Physicians and Surgeons, New York, NY, USA, 3SUNY Downstate College of Medicine, New York, NY, USA, 4Tufts University, Boston, MA, USA, 5Weill Cornell Medicine, Department of Surgery, New York, NY, USA.
Background: The study of breast cancer (BC) is limited by experimental paradigms which fail to address the complexity of the tumor microenvironment and inherent patient-to-patient variability, both in the tumor and its microenvironment. To overcome these challenges, we developed a revolutionary three-dimensional ex vivo culture system which allows for assemblage of patient-derived cell types in their proper anatomic arrangement. Using this system, we can determine the relative aggressiveness of tumors and the effect of the local tumor microenvironment by assessing their ability to drive Human Umbilical Vein Endothelial Cell (HUVEC) invasion, a crucial step required for tumors to metastasize.
Methods: Patient-derived breast tissue was processed to isolate adipocytes, stromal cells, and organoids. These components were mixed with 0.3% nonribosylated (NR) or ribosylated Type I collagen. Fluorescently tagged MDA-MB-231 and MDA-MB-468 triple negative BC cells were plated at a density of 5k cells (hereafter referred to as “cancer button”) at the center of each well. A layer of Type I collagen and a monolayer of HUVECs were also plated. The eight groups consisted of: four experimental groups (NR collagen only, NR biomimetic platform, ribosylated collagen only, and ribosylated biomimetic platform), with two subgroups each (cancer buttons with a HUVEC monolayer and HUVEC monolayer alone). Each well was imaged via confocal microscopy on days 0, 4, and 7. Analysis was performed using Imaris™ software.
Results: This study demonstrates tumor-induced HUVEC migration towards the cancer button over seven days (Figure 1), as compared to the near complete lack of invasion in control groups containing a HUVEC monolayer alone (Figure 1A). The data also show a statistically significant increase in HUVEC invasion of the cancer button, with an average increase in HUVEC percentage within the button of 4.2% between days four and seven (p=0.0002) in the NR collagen only groups. This is compared to an average increase of 3.1% in the NR biomimetic groups (p<0.0001), and 0.9% in the ribosylated biomimetic groups (p<0.0001). The cancer cells additionally showed a 52% increase in cell count over seven days.
Conclusions: We have demonstrated both migration of HUVECs toward the BC buttons as well as a statistically significant increase in percentage of HUVECs invading each cancer button within our platform. These data demonstrate that tumor induction of angiogenesis is modulated by the local microenvironment and that the relative aggressiveness of tumor tissue, as indicated by the degree of angiogenic induction, can be assayed using this novel patient-specific platform. 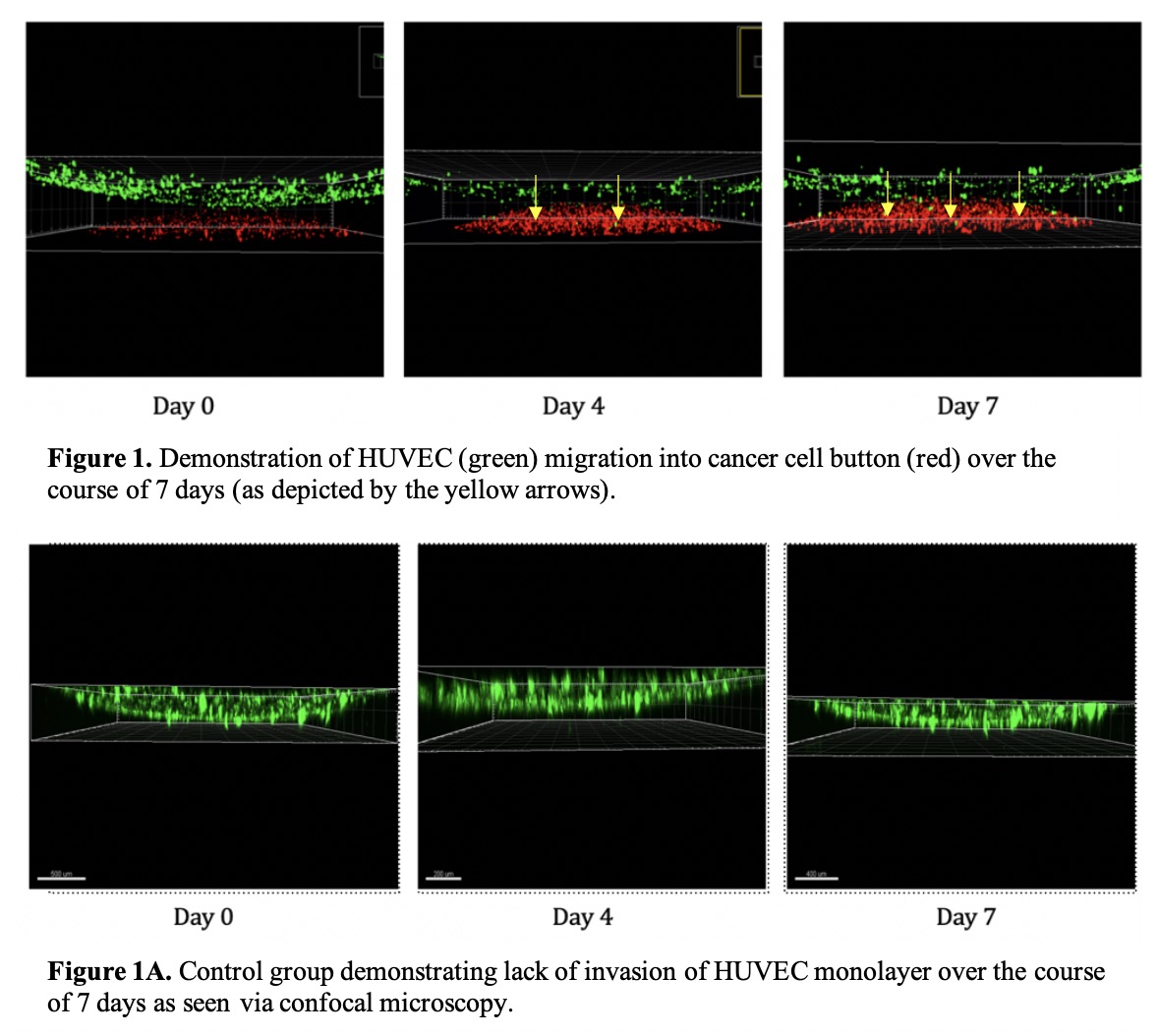
Back to 2019 Abstracts
