Assessment of Opioid Prescribing Practices in Breast Augmentation: Future Directions for Prescribing Guidelines
Dustin T. Crystal, B.S.1, Nicholas G. Cuccolo, B.S.1, Michael J. Plewinski, PharmD1, Ahmed MS Ibrahim, MD, PhD1, Jeremy C. Sinkin, MD2, Samuel J. Lin, MD, MBA1, Richard L. Agag, MD2, Bernard T. Lee, MD, MBA, MPH1.
1Beth Israel Deaconess Medical Center, Boston, MA, USA, 2Rutgers-Robert Wood Johnson Medical School, New Brunswick, NJ, USA.
BACKGROUND: The United States (U.S.) is in the midst of an opioid epidemic propagated, in part, by prescription opioids. With excess overprescribing documented in a variety of surgical procedures, several societies have recommended opioid-prescribing guidelines. Considering the scope and post-operative pain associated with aesthetic plastic surgery procedures, earnest evaluation into opioid prescribing practices for breast augmentation was conducted.
METHODS: Members of the American Society for Aesthetic Plastic Surgery (ASAPS) were electronically surveyed on their opioid prescribing patterns. The survey was distributed to 1,709 attending plastic surgeons. Descriptive statistics were collated into percentages, deviations, and morphine milligram equivalents (MME), when appropriate.
RESULTS: 229 (13.4%) ASAPS members provided responses. 91.2% of respondents prescribe opioids to patients undergoing breast augmentation. The most commonly prescribed agents included oxycodone/acetaminophen (Percocet; 47.0%) and hydrocodone/acetaminophen (Vicodin; 38.3%). On average, 165.3±81.7 MME were dispensed (range: 25.0-600.0 MME; number tablets: 5-60). The reported pre-, intra-, and post-operative adjuvant modalities utilized as means to spare-opioids were equally variable (Figure/Table 1). Presented with evidence-based data on patient use of opioids following secondary breast reconstruction or breast reduction procedures (11-18 tablets of oxycodone 5.0 mg/acetaminophen 325 mg), respondents noted that they would comfortably prescribe patients 17.9+/-9.0 tablets (range 0-60 tablets). Prescribers felt that a lack of phone-in prescribing (52.4%) and the ease of preemptively prescribing opioids (52.4%) propagate opioid overprescribing. 61.3% of respondents reported that they are or may be in favor of developing plastic surgery societal guidelines related to opioid prescribing. These respondents indicated support for guidelines on opioid-sparing pain management strategies (74.2%) and guidelines identifying the type (54.7%), duration of use (69.5%), and number of opioid tablets (61.7%) necessary for procedures. Comparatively, 35.4% of respondents reported that societies should not develop such guidelines, largely citing that patients/procedures are individualized and warrant individual prescribing on a case-by-case basis (41.9%).
CONCLUSIONS: Considerable variability exists among prescribing patterns following breast augmentation. Societal guidelines aimed at providers and patients may serve a future role in opioid prescribing. 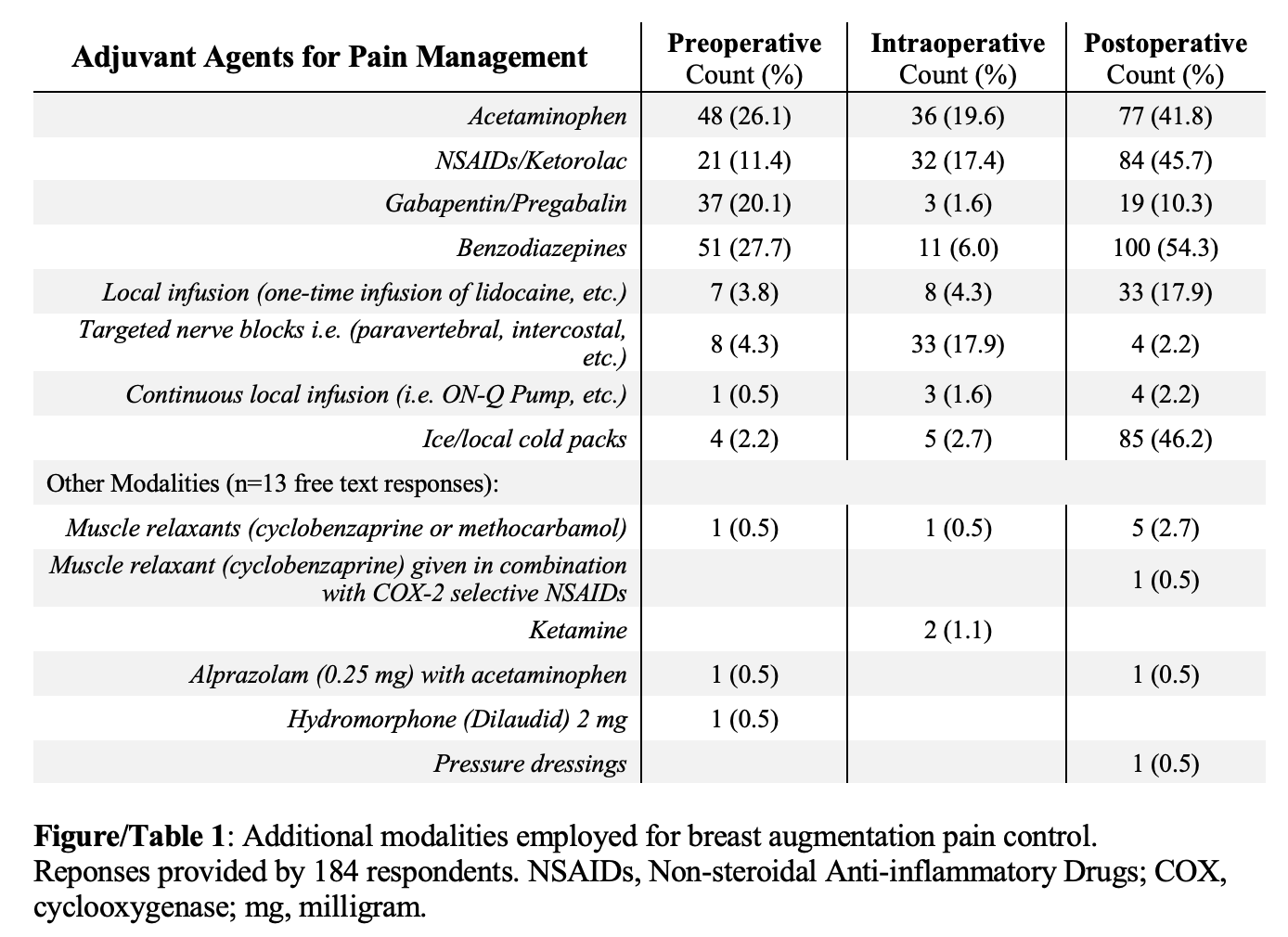
Back to 2019 Posters
