Micropuncture Induced Scaffold Vascularization and Macrophage Quantification Across Vascular Origins
Summer N. Horchler1, Sonakshi Sharma1, Jessica Collins1,2, John Roberts2, Mingjie Sun1,2, Srinivas V. Koduru1,2,3, Dino J. Ravnic1,2
1Irvin S. Zubar Plastic Surgery Research Laboratory, Penn State College of Medicine, Hershey, PA, USA2Department of Surgery, Penn State Health Milton S. Hershey Medical Center, Hershey, PA, USA3Department of Cellular and Molecular Physiology, Penn State College of Medicine, Hershey, PA, USA
Purpose: Vascularization is a major barrier to tissue engineering and macrophages are believed to be integral to angiogenesis. Recently, we described a microsurgical approach which rapidly vascularizes an adjacently placed scaffold. In micropuncture (MP), the recipient blood vessel wall is precisely disrupted to provide an immediate route for cell extravasation. Here we evaluated the association of resulting scaffold vascularization and macrophage infiltration when MP was applied to the artery alone, vein alone, and both the artery and vein. We hypothesized that concurrent artery/vein MP would result in greatest scaffold macrophage infiltration and vascularization.
Methods: MP was tested in the rat femoral artery (MPA), femoral vein (MPV), femoral artery and vein (MPA/V), and no MP (control). 60-um diameter MPs were created at 1 mm intervals over a 15 mm length, just prior to adjacent implantation of a Type 1 collagen scaffold. At post-operative day (POD) 3 and 10, animals underwent in situ fluorescence angiography. Samples were then prepared for both whole mount and thin section histology. Vascular density (vessel area/total area) was computed using artificial intelligence (AI; MetaVi Labs; Austin, TX). Endothelial cell (CD31) and macrophage (F4/80) infiltration was quantified using immunofluorescence staining and ImageJ analysis. Statistical significance was defined as p<0.05.
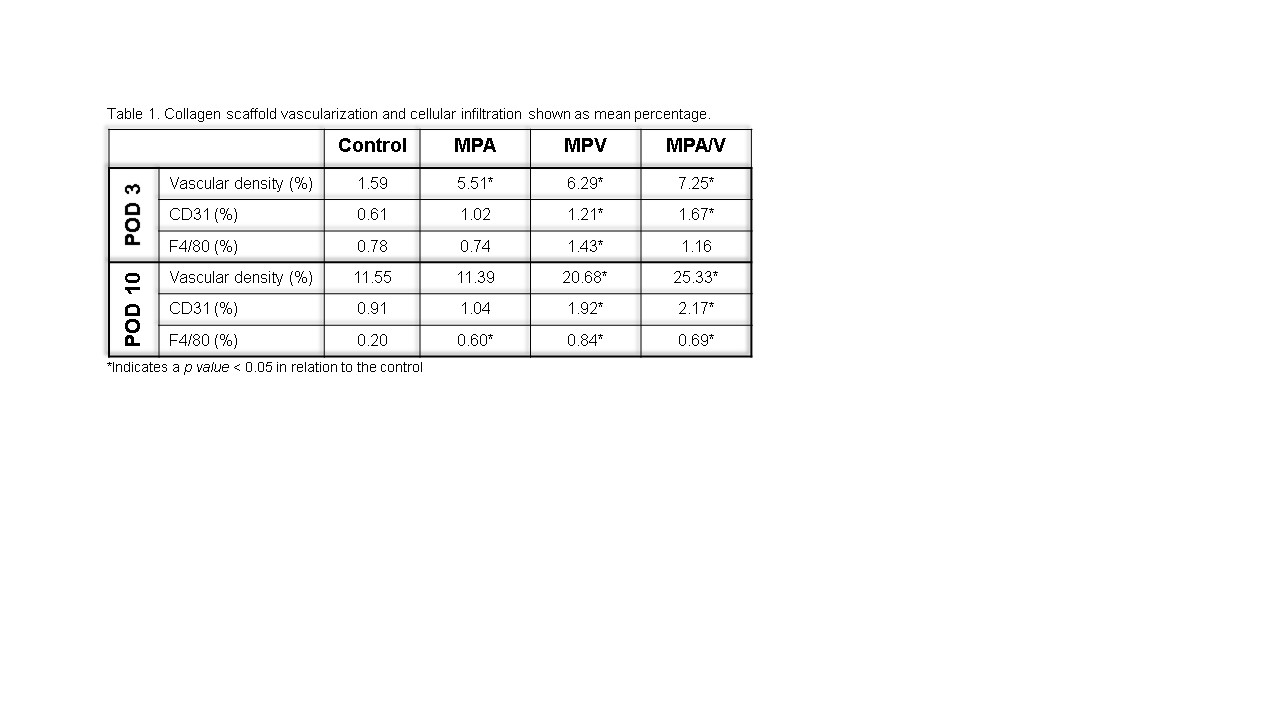
Results: Results are detailed in Table 1. Scaffold vascular density was concordant with increased endothelial cell infiltration at all timepoints under virtually all scenarios. MPA/V resulted in the greatest vascular density and endothelial cell infiltration followed by MPV and MPA. However, the greatest amount of macrophage infiltration was noted in MPV at both timepoints followed by MPA/V. Both conditions resulted in a more rapid rise in macrophage accumulation when compared to MPA and the control. Macrophage counts had largely equilibrated by POD 10 in all groups.
Conclusion: Micropuncture appears to create a localized pro-angiogenic environment. Although concurrent MP of the artery and vein leads to the greatest degree of scaffold vascularization, our findings are noteworthy that MP of the vein alone is also substantially pro-angiogenic. It appears that the accelerated macrophage infiltration observed is associated with MP of the vein, either alone or with arterial combination. This leads us to believe that venous MP is the primary driver of both the changes in the cellular microenvironment and resultant scaffold vascularization. Future, studies are planned to evaluate this in more detail. Nevertheless, MP appears to offer a tailored surgical approach for angiogenic induction which may prove beneficial to tissue engineering.
Back to 2022 Abstracts