Augmenting Breast Implant Research: Accessible Methods for Fabricating Miniature Smooth and Textured Breast Implants in a Laboratory Setting
Sabrina Shih1, Carly Askinas2, Nicholas Vernice3, Luke Poveromo3, Hector Salazar Martinez3, Xue Dong3, Jason Spector3
1Columbia University, New York, NY 2Tulane University, New Orleans, LA, 3Weill Cornell Medical College, New York, NY
BACKGROUND: Due to the association of textured breast implants with breast implant associated anaplastic large cell lymphoma (BIA-ALCL), anatomically shaped breast implants have been voluntarily recalled from the US breast implant market, which rely on the textured surface of implants to maintain rotational stability. Although preclinical studies on breast implant technology have traditionally been undertaken by commercial breast manufacturers due to the complexities of implant manufacturing, we have developed miniature-scale breast implants constructed of polydimethylsiloxane (PDMS) that allow for high-throughput prototyping and testing in a laboratory setting.
METHODS: Two-piece positive molds for a dome-shaped implant measuring 2 cm by 1 cm were constructed in Fusion360 and printed in Polysmooth filament using a Prusa i3 MK2S+ 3D printer. Molds for smooth implants and implants with pores for tissue ingrowth measuring 2mm or 4 mm in diameter and depth for tissue ingrowth were cycled through five smoothing and drying intervals using 200-proof ethyl alcohol. Textured molds were painted with a 2.2:1 ratio of fine sugar to an epoxy resin by weight. Implant molds were filled with a 20:1 silicone to curing agent ratio of PDMS, internally marked with colored PDMS to indicate directionality, and cured at 37C for 24 hours. Implants were sputter coated with 5 nm of platinum using a Leica EM Sputter Coater and imaged using an Electron Optics Instruments Cube II SEM. Allergan Bio-CELL, Allergan smooth, and Sientra smooth implant shells were used as controls for imaging.
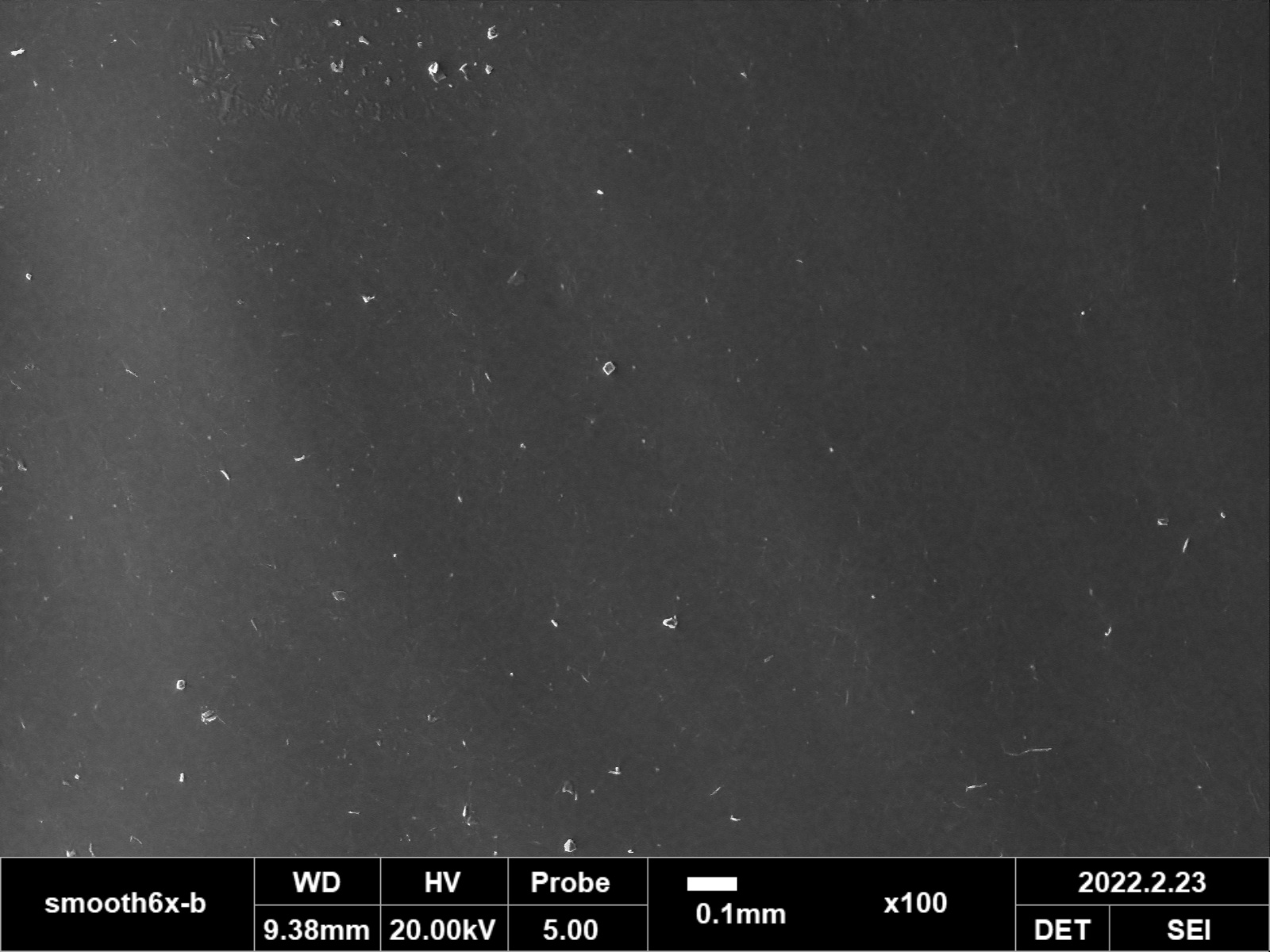
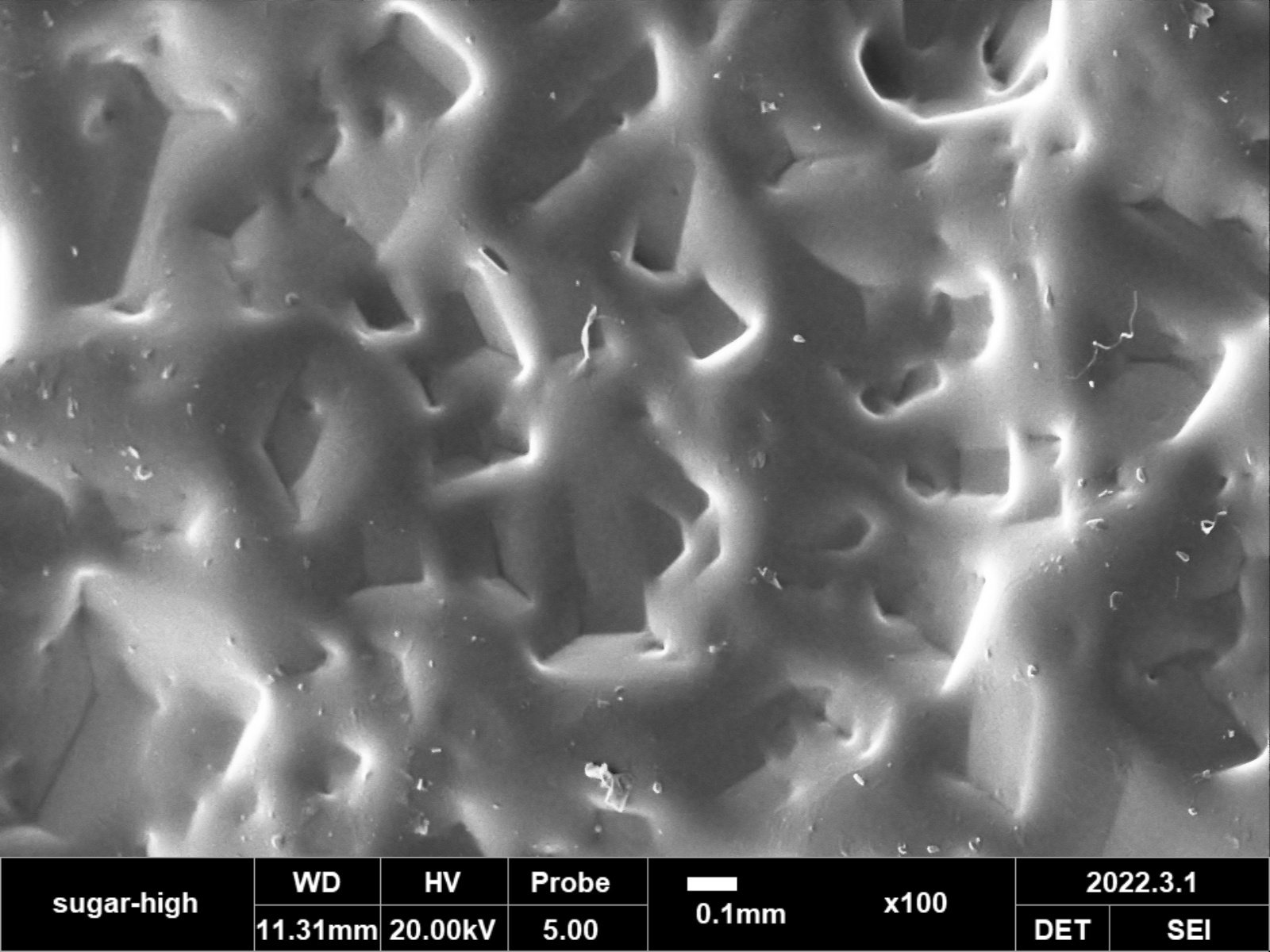
RESULTS: Implants retained the original dome shape of the 3D printed molds. Qualitative assessment of SEM images at 100X, 200X, and 500X demonstrated similar surface topography between our smooth PDMS implants, implants with pores for tissue ingrowth, and commercially available smooth implant shells from Allergan and Sientra. For the textured groups, there was no statistical difference in the size of the surface indentations comprising the texture measured from the Allergan Bio-CELL implant compared to our textured implants (p<0.05). There was also no statistically significant difference in the number of indentations between groups, with 28.2±1.4 indentations per 100X field for the Allergan textured group and 27.5±-0.7 in our textured implants.
CONCLUSION: This study demonstrates a low-cost customizable approach to fabricating smooth and textured breast implant models fit for short and long-term implant studies in animals. The accessibility of this implant fabrication strategy will allow for both industry and non-industry investigators to more rapidly develop preclinical and clinical models of shaped implants for pre-clinical investigation.
Back to 2022 Abstracts