Fabricating A Customizable, Low-cost Vascular Tissue Flap
Carly Askinas B.S.1, Nick Vernice A.B.1, Sabrina Shih B.A.1, Ryan Bender B.S.1, Xue Dong M.D. Ph.D.1, Hector Salazar Martinez B.S.1, Jason A. Spector M.D.1
1Laboratory of Bioregenerative Medicine & Surgery, Weill Cornell Medicine, New York, NY
Background: Reconstructive surgeons rely heavily on autologous donor tissue as a source of replacement tissue, which consequently results in donor site morbidity. Efforts to solve this problem by engineering soft tissue are constrained in size by their reliance on vasculature, as constructs at thicknesses greater than 100-200 um exceed the maximum nutrient diffusion distance for cell survival and require their own blood supply. Currently, research is focused on the inclusion of mature microvascular networks within tissue constructs, however, their small scale is not readily clinically translatable. Here, we present the creation of a customizable tissue construct with a larger-scale vascularized channel that is compatible with a low-cost perfusion chamber and has the potential for surgical anastomosis to recipient vasculature.
Methods: Tissue constructs are housed in a custom PDMS base and PLA frames compatible with our perfusion chamber. Fabrication was completed using 3D-modeling software (Fusion 360) and a 3D printer (Prusa i3 MK3S) in PLA. Vascularized channels (1.5 mm in diameter) were created by the sacrifice of custom Pluronic F127 loops in 1% type I collagen. Channels were flushed with 20mL PBS at 1mL/min to remove residual Pluronic. The channels were sequentially seeded with RFP Smooth Muscle Cells (SMCs) and GFP E4 Endothelial Cells (ECs). Perfusion devices were maintained in static culture for 3 days then either perfused for 5 days (2 mL/min) or maintained in static culture for another 7 days. Imaging was performed with fluorescent microscopy. Histologic sectioning and immunofluorescence were also performed.
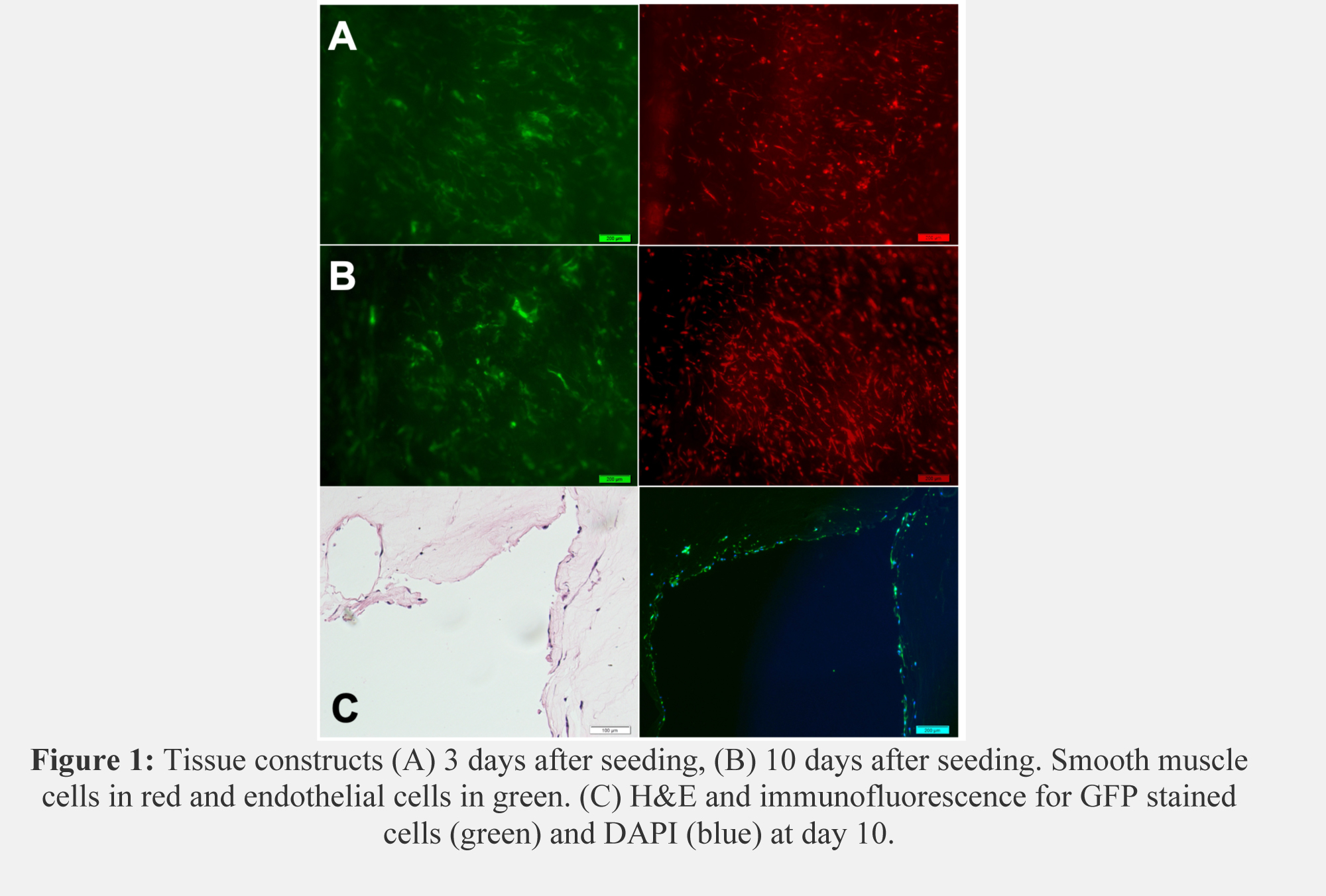
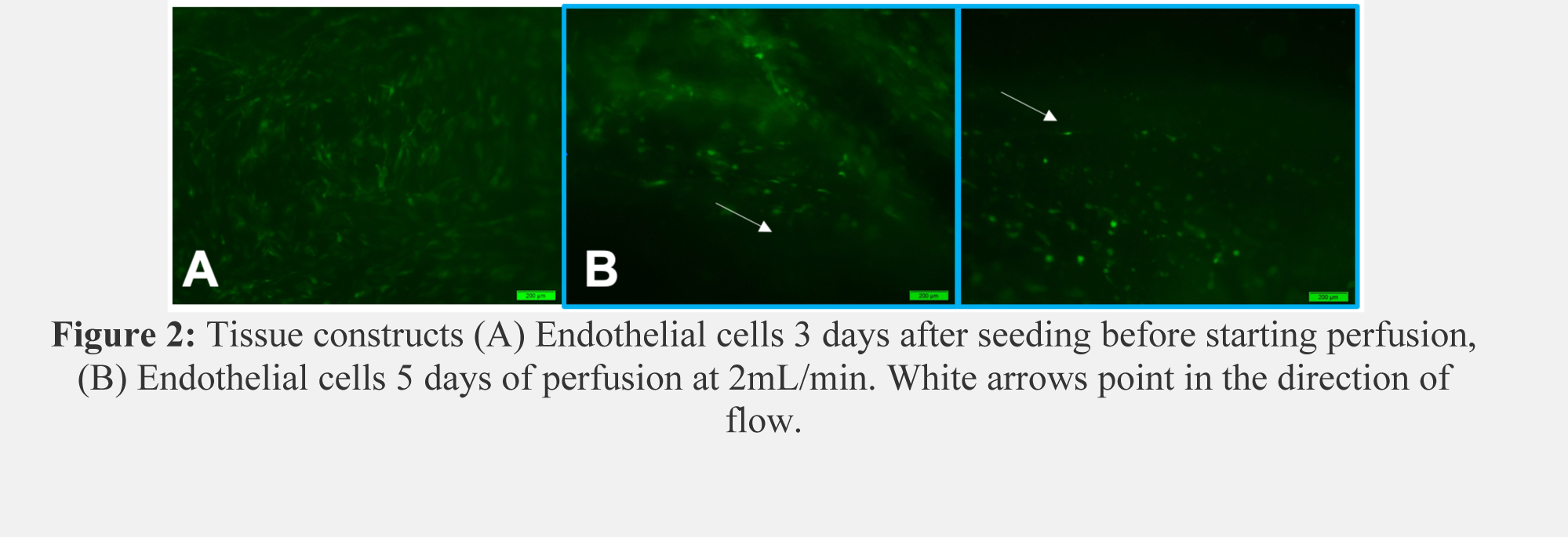
Results: Microscopy revealed proper cell seeding with confluent ECs and SMCs. After 10 days in static culture, collagen loops maintained high cell viability with elongation of SMC morphology (Figure 1A, B). Histology exhibits cell-lined channels and immunofluorescence indicates most of the lining is composed of GFP-ECs. (Figure 1C). After 5 days of perfusion, ECs and SMCs aligned with the direction of flow and began to form a barrier at the collagen edges (Figure 2).
Conclusion: This large-scale perfusable tissue construct holds great potential for the field of microvascular tissue engineering. This work shows proof of concept of a large vascular structure embedded in a collagen ECM offering researchers the potential to create a vascular channel with the design of their choice, a cell-laden or non-cell-laden collagen ECM, and compatibility with a low-cost perfusion circuit. This device may be replicated and customized to individual needs, making it very valuable for translational research laboratories.
Back to 2022 Abstracts