Outcomes After Implant-Based Breast Reconstruction Following the National Institution of a Ban on Bacitracin Irrigation
Nikita Roy*, Olachi Oleru, Daniel Y. Kwon, Anya Wang, Catherine Stratis, Arya A. Akhavan, Uchechukwu O. Amakiri, Anais Di Via Ioschpe, Martina Brozynski, Peter Henderson
Icahn School of Medicine at Mount Sinai, New York, NY
The use of irrigation with bacitracin-containing solution has been common among surgeons, as it was widely thought to have anti-bacterial properties and to prevent post-operative infection. On January 31, 2020, the Food and Drug Administration (FDA) instituted a ban on bacitracin-containing irrigation for operative use. This study aimed to determine whether bacitracin has a beneficial effect on postoperative infection rates by analyzing infection rates before and after the FDA ban on bacitracin irrigation.
A single-institution retrospective chart review was conducted. Eligible patients underwent implant-based breast reconstruction following total mastectomy. Procedure date, reconstruction type, patient comorbidities, use of bacitracin irrigation, post-operative infection, and secondary outcomes were collected. Univariate and multivariable logistic regression analysis was performed.
A total of 188 female patients aged 30-83 were included in the study, who underwent a total of 345 procedures. Twenty-seven (14%) pre-ban procedures resulted in post-operative infection and 21 (14%) post-ban procedures resulted in post-operative infection. Nine (13%) procedures involving bacitracin irrigation use resulted in a post-operative infection; of these procedures, 4 took place prior to the bacitracin ban and 5 took place after the bacitracin ban. Bacitracin use did not protect against infection in univariate or multivariable analysis. Age over 50 years was associated with increased risk of postoperative infection (p=0.03). The presence of comorbidities, smoking status, neoadjuvant chemotherapy, implant position prepectoral versus subpectoral, and laterality were not statistically significantly associated with postoperative infection development.
The use of bacitracin-containing irrigation solution does not decrease the risk of postoperative infection. As bacitracin is no longer FDA approved for use in irrigation, further research is required to explore the optimal antibiotics for inclusion in pocket irrigation for implant-based reconstruction.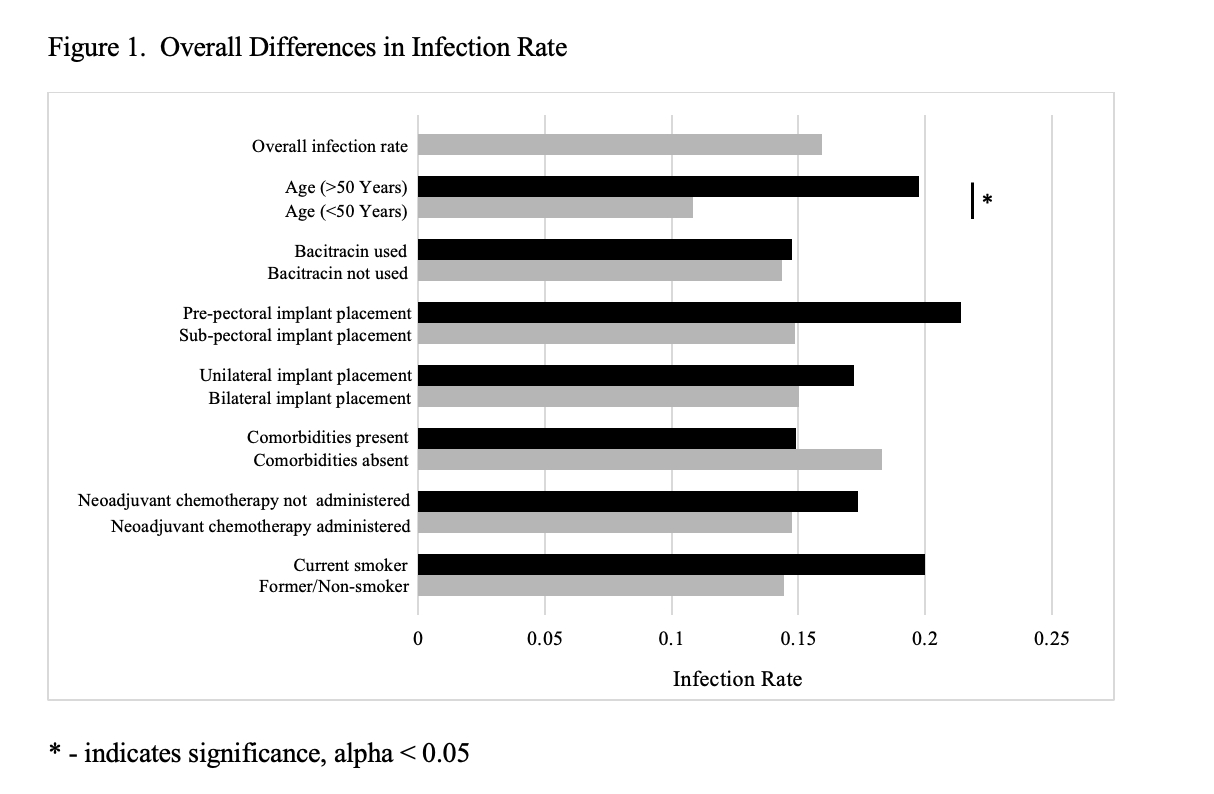
Back to 2023 Abstracts


