Customizable, Low-cost, Biovital Implants for Craniofacial Reconstruction
Gillian O’Connell*, George S. Corpuz, Hector Salazar Martinez, Nicholas A. Vernice, Xue Dong, Jason A. Spector
Laboratory of Bioregenerative Medicine & Surgery, Division of Plastic Surgery, Weill Cornell Medical College, New York, NY
Craniofacial reconstruction requires autologous, cadaveric or alloplastic implants and intraoperative modification to fit the aesthetic needs of each patient. Alloplastic implants introduce risks of infection, extrusion, expense and donor site morbidity. There is substantial need for customizable and low-cost facial implants that can induce neotissue formation. This study examines the biocompatibility of polylactic acid (PLA) implants +/- decellularized cartilage infill to assess translatability to craniofacial (nasal) reconstruction.
Dorsal nasal scaffolds were designed with 3D modeling software and printed in PLA. Two scaffolds designed as external "cages"ť without internal supports were filled with decellularized cartilage harvested from ovine ribs. The remaining two scaffolds had internal PLA rebar and were implanted without infill. Scaffolds were heterotopically implanted on rat dorsa with 4 implants per rat. Scaffolds were explanted 3 and 6 months and underwent same-day volumetric analysis via microCT followed by histopathologic and immunohistochemical analysis.
Empty and zested cartilage-filled cages had significant volume loss at 3 and 6 months relative to volume at implant with explanted constructs grossly collapsed at both timepoints (p < 0.05). Volume retention in rebar and minced cartilage-filled constructs was superior to empty and zested-filled scaffolds at 6 months. Hematoxylin and eosin stain showed a subsiding inflammatory response and organized fibrovascular neotissue deposition at 6 months; immunofluorescence revealed an M2-dominant macrophage phenotype.
This study supports the use of PLA for generating customizable facial implants. With sufficient internal support, constructs retain contours and volume at 6 months post-implant and provide a scaffold for ingrowth of healthy, vascularized, collagen-rich tissue. Design and fabrication these implants can be completed quickly and at minimal cost, potentially at the point of care, allowing for affordable biovital implants with a minimal risk profile.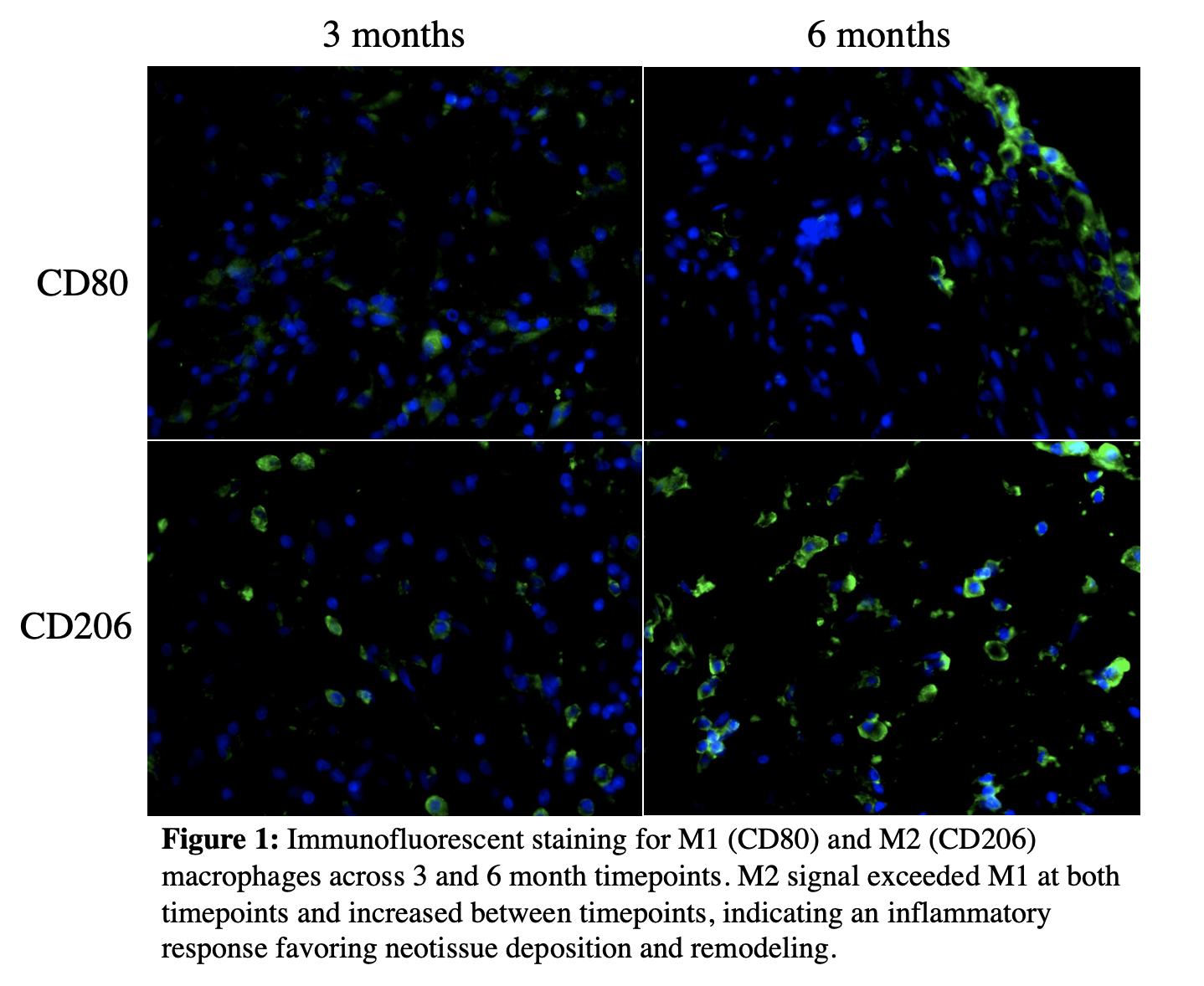
Back to 2023 Abstracts


