Outcomes of Nipple-sparing Mastectomy with Reconstruction after Recent Oncoplastic Wisepattern Reduction
Salman Choudhry*1, Tasha Martin2, Tripp Holton1, Rubie S. Jackson2
1Division of Plastic Surgery, Anne Arundel Medical Center, Annapolis, MD; 2Department of Breast Surgery, Anne Arundel Medical Center, Annapolis, MD
For patients with large and/or ptotic breasts, a planned staged approach to nipple-sparing mastectomy (NSM) has been described. Less is known about surgical outcomes of unplanned staged NSM for management of positive margins after partial mastectomy with oncoplastic reduction. It is not clear from earlier studies whether an interval of less than 10 weeks between oncoplastic reduction and NSM is feasible, when a shorter interval is important for oncologic reasons.
This is a single institution analysis of patients from 2018 to 2021 with a diagnosis of invasive cancer or ductal carcinoma in situ who underwent NSM after oncoplastic breast reduction for positive margins or nodes. The primary endpoint measured was nipple loss. Secondary outcomes were need for operative re-intervention and wound complications.
Nine patients (14 breasts) underwent partial mastectomy with oncoplastic Wise-pattern breast reduction, followed by NSM. Three patients underwent intersurgery chemotherapy. The average interval between oncoplastic reduction and NSM was 11.3 weeks when excluding patients undergoing chemotherapy (range 8-13 weeks). Thirteen breasts (93%) underwent pre-pectoral direct-to-implant reconstruction. One breast (7%) received autologous reconstruction. One breast required reoperation for seroma. The rate of partial or total nipple loss was 0%, with an average follow-up of 1.6 years.
Our experience demonstrates excellent outcomes from NSM after oncoplastic breast reduction, with the majority of patients undergoing single-stage pre-pectoral direct-to-implant breast reconstruction. Overall, patients had a shorter intersurgery interval, compared with prior studies, with no cases of nipple loss. An intersurgery interval of 8 weeks may be feasible when avoiding delays is important for oncologic reasons.
Table 1. Patient Characteristics and Tumor Type
| Patient | Age At Diagnosis | BMI | Average SNTND (cm) | Breast Cup Size | Breast Involved | Quadrant | Tumor Type by Excision | Smoking Status | Free Nipple Graft | Follow Up (mo.) |
| 1 | 44 | 31 | 29 | 38D | Right | UO | DCIS, IDC | Never | No | 27.5 |
| 2 | 57 | 40 | 41 | 42DDD | Right | LO | DCIS, IDC | Never | Yes (U) | 25.4 |
| 3 | 38 | 49 | 42 | 44F | Right | UI | DCIS, IDC | Former | Yes (B/L) | 23.7 |
| 4 | 43 | 30 | n/a | 34DDD | Right | UO | DCIS, IDC | Never | No | 24.3 |
| 5 | 46 | 22 | 24 | n/a | Left | LI | DCIS, IDC | Never | No | 2.0 |
| 6 | 58 | 32 | 29 | 42A | Right | UO | IDC | Former | No | 21.8 |
| 7 | 50 | 35 | 33 | 40DDD | Left | UO | Mixed IDC | Never | No | 2.8 |
| 8 | 52 | 22 | 24 | 36B | Right | UI | DCIS, IDC | Never | No | 0.3 |
| 9 | 55 | 31 | 29 | 36C | Left | UO | ILC | Never | No | 5.2 |
DCIS, Ductal Carcinoma in Situ; IDC, Invasive Ductal Carcinoma; Invasive Lobular Carcinoma; SNTND, Sternal Notch to Nipple Distance; UO, Upper outer; UI, Upper Inner; LO, Lower Outer; LI, Lower Inner; Unilateral, U; Bilateral, B/L
Table 2: Interval Between Oncoplastic Reduction and Method of Reconstruction
| Patient | Mastectomy | Weeks Between Reduction and NSM | Chemotherapy | Method of Reconstruction | Tissue Perfusion Assessment (SPY) | Dressing | Implant Size (cc) | Partial Mastectomy Weight (g) with Margins | Reduction Specimen Weight (g) | Left Breast Mastectomy Weight (g) | Right Breast Mastectomy Weight (g) |
| 1 | Unilateral | 13 | None | DIEP | No | Prevena | n/a | 69 | 89 | Unilateral | 594 |
| 2 | Bilateral | 20 | Neoadjuvant | pDTI | Yes | Prevena | 800 | 517 | 935 | 1229 | 885 |
| 3 | Unilateral | 8 | Adjuvant | pDTI | Yes | Prevena | 800 | 108 | 1818 | Unilateral | 1254 |
| 4 | Bilateral | 21 | Neoadjuvant | pDTI | Yes | Conventional | 800 | 245 | 747 | 535 | 703 |
| 5 | Bilateral | 29 | Neoadjuvant | pDTI | Yes | Bella | 495 | 26 | 14 | 432 | 406 |
| 6 | Unilateral | 11 | Adjuvant | pDTI | Yes | Bella | 485 | 35 | 88 | Unilateral | 385 |
| 7 | Unilateral | 13 | None | pDTI | Yes | Bella | 650 | 230 | 540 | 686 | Unlateral |
| 8 | Bilateral | 12 | None | pDTI | Yes | Bella | 240 | 1720 | 155 | 189 | 202 |
| 9 | Bilateral | 11 | Adjuvant | pDTI | Yes | Bella | 520 | 109 | 382 | 374 | 440 |
DIEP, Deep Inferior Epigastric Perforator. pDTI, Pre-pectoral Direct To Implant
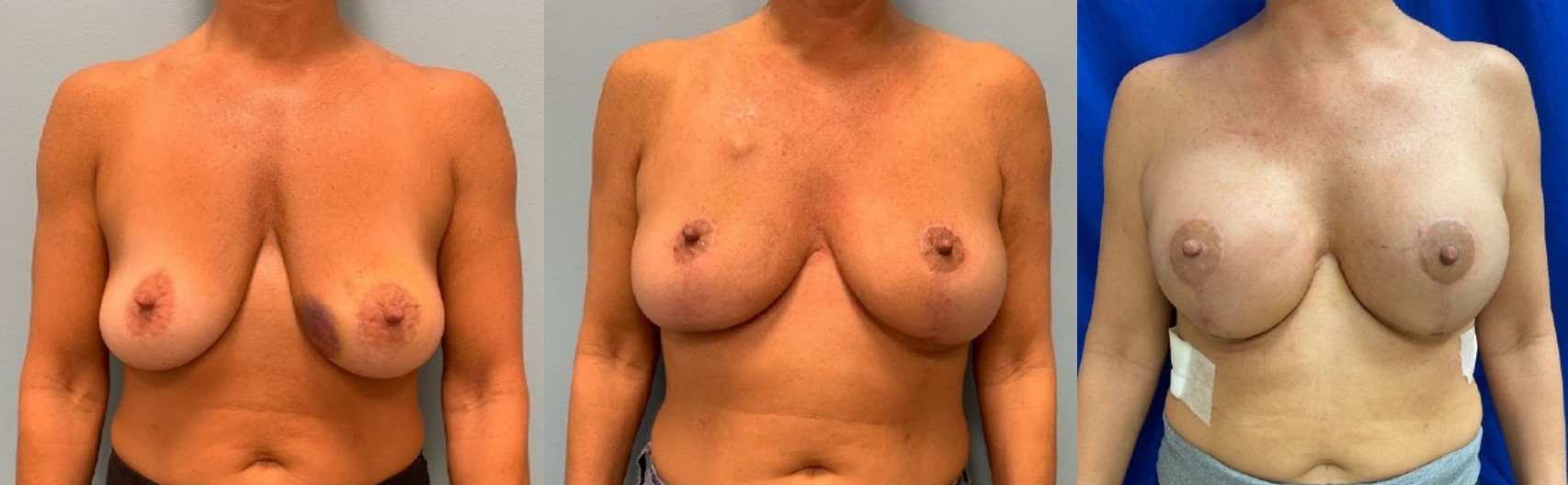
Clinical photos, patient 5. A, Pre-oncoplastic breast reduction of patient 5 after core needle biopsy with left-sided breast cancer, grade II ptosis and a body mass index (BMI) of 22 kg/m2. B, The patient 3 months postoncoplastic breast reduction. C, The patient 2 weeks post-NSM with pDTI breast reconstruction and placement of 495 cm3 extra profile implants. The patient received chemotherapy between oncoplastic reduction and NSM, resulting in a 29-week interval between reduction and NSM.
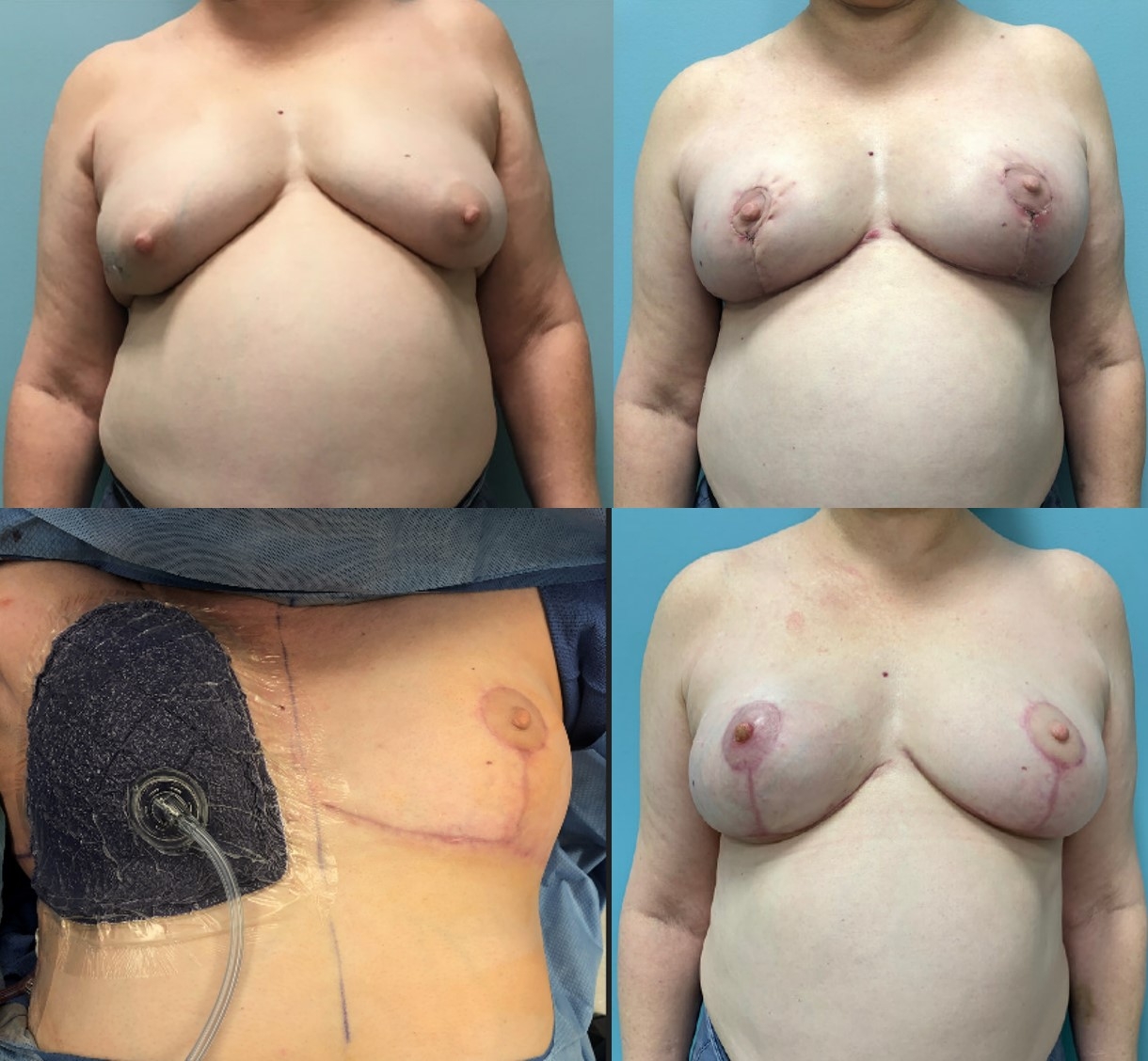
Clinical photos, patient 6. A, Pre-oncoplastic breast reduction of patient 6 with right sided breast cancer, grade II ptosis, and BMI of 32 kg/m2. B, The patient 16 days postoncoplastic breast reduction. C, The patient with placement of Prevena Restor BellaForm dressing which was removed at the first postoperative visit. D, The patient 16 days post right NSM with pDTI breast reconstruction and placement of 485 cm3 full profile implants. Interval of 11 weeks between breast reduction and NSM. Postoperatively, the patient developed mild right nipple epidermolysis, which healed by conservative measures.
Back to 2023 Abstracts


