Micropuncture Vascularization of Intraoperative Bioprinted Scaffolds
Summer N. Horchler*1, Miji Yeo2, Dishary Banerjee2, Olivia Waldron1, Jessica C. El-Mallah1, Mingjie Sun1, Ibrahim T. Ozbolat2, Dino J. Ravnic1
1Irvin S. Zubar Plastic Surgery Research Laboratory, Penn State College of Medicine, Hershey, PA; 2Department of Engineering Science and Mechanics, Penn State University, State College, PA
Backgroud:
Tissue loss is a major cause of worldwide morbidity and mortality, and plastic surgeons are tasked with its correction. Intraoperative bioprinting (IOB) represents the next generation of reconstructive surgery. Although promising, translation and scalability are limited by poor recipient vascularization. Recently, we described a microsurgical approach which rapidly vascularizes an adjacently placed scaffold. In micropuncture (MP), the recipient blood vessel wall is precisely disrupted to provide an immediate route for cell extravasation. We hypothesized that MP integration with IOB would improve scaffold vascularization.
Methods:
MP was tested in the rat femoral artery and vein (n=5) with control animals receiving no MPs (n=5). Immediately after, an extrusion bioprinter was brought into the operative field and deposited a collagen-based scaffold directly over the femoral vessels. At Day 10 animals underwent in situ laser doppler and fluorescence angiography. Samples were analyzed using whole mount angiography and histology. Vessel metrics were analyzed using artificial intelligence. Endothelial (EC; CD31) and nucleated cells (DAPI) were quantified.
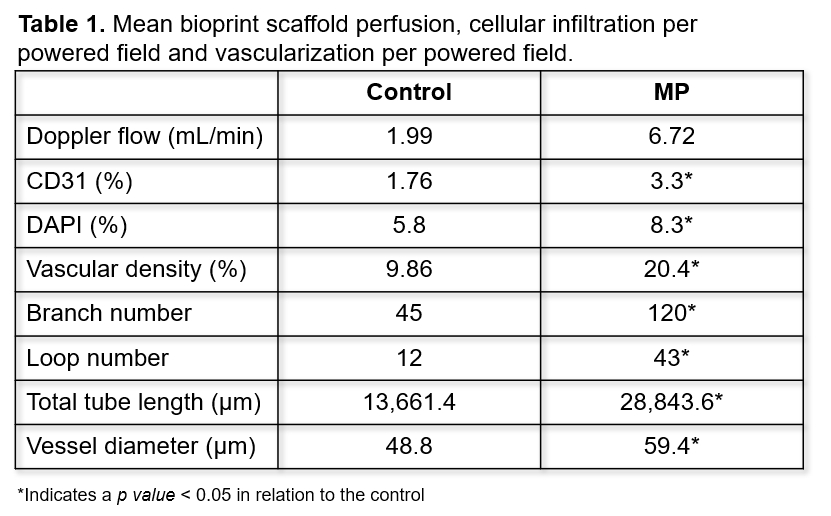
Results:
A 3-fold increase in MP scaffold perfusion was seen at Day 10 although this did not reach statistical significance. An increase in EC and nucleated cell counts was seen in MP scaffolds. Vascular density showed a 2-fold increase in MP scaffolds when compared to controls. Capillary network expansion was attributed to increased branch numbers within MP scaffolds resulting in more vascular loop formation. Increases in tube length and diameter were also seen in MP scaffolds. (Table 1)
Conclusion:
Here we demonstrate the simple integration of microsurgery with IOB as a platform approach for engineered tissue vascularization. MP appears to be suitable with collagen-based bioinks and creates a pro-angiogenic microenvironment. Ongoing work will determine the feasibility of also introducing cells into the bioprinted scaffold with the long-term goal of building in-situ flaps for tissue reconstruction.
Back to 2023 Posters


